Payload Information
General Information of This Payload
| Payload ID | PAY0JCIBW |
|||||
|---|---|---|---|---|---|---|
| Name | Mertansine DM1 |
|||||
| Synonyms |
Mertansine; Maytansinoid DM 1; 139504-50-0; maytansinoid DM1; Mertansine (DM1); N2'-deacetyl-N2'-(3-mercapto-1-oxopropyl)-maytansine; DDZ29HGH0E; DM 1; [(1S,2R,3S,5S,6S,16E,18E,20R,21S)-11-chloro-21-hydroxy-12,20-dimethoxy-2,5,9,16-tetramethyl-8,23-dioxo-4,24-dioxa-9,22-diazatetracyclo[19.3.1.110,14.03,5]hexacosa-10,12,14(26),16,18-pentaen-6-yl] (2S)-2-[methyl(3-sulfanylpropanoyl)amino]propanoate; DM1; Maytansine, N2'-deacetyl-N2'-(3-mercapto-1-oxopropyl)-; N2'-Deacetyl-N2'-(3-mercapto-1-oxopropyl)-maytansine, L-; DM1 [Maytansinoid]; DM 1 [Maytansinoid]; UNII-DDZ29HGH0E; Mertasine; DM1;Maytansinoid; Maytansinoid DM1; MAYTANSINOID DM1 [MI]; CHEMBL4802230; SCHEMBL13558634; CHEBI:82755; MFCD28398157; s6773; CS-5804; DA-48536; HY-19792; J3.653.420F; Q4515649; (1S,2R,3S,5S,6S,16E,18E,20R,21S)-11-chloro-21-hydroxy-12,20-dimethoxy-2,5,9,16-tetramethyl-8,23-dioxo-4,24-dioxa-9,22-diazatetracyclo[19.3.1.1(10,14).0(3,5)]hexacosa-10(26),11,13,16,18-pentaen-6-yl (2S)-2-[methyl(3-sulfanylpropanoyl)amino]propanoate; (3S)-3-O-De[2-(acetylmethylamino)-1-oxopropyl]-3-O-[(2S)-2-(methyl 3-mercaptopropanoylamino)propanoyl]maytansine
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
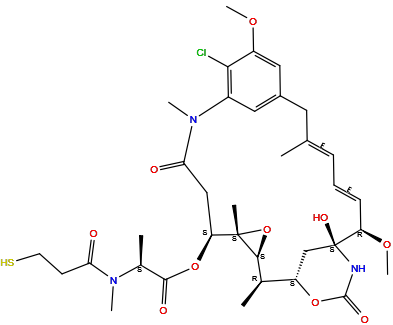
|
|||||
| Formula | C35H48ClN3O10S |
|||||
| Isosmiles | [H]O[C@@]12N([H])C(=O)O[C@@]([H])(C1([H])[H])[C@@]([H])(C([H])([H])[H])[C@]1([H])O[C@@]1(C([H])([H])[H])[C@@]([H])(OC(=O)[C@@]([H])(N(C(=O)C([H])([H])C([H])([H])S[H])C([H])([H])[H])C([H])([H])[H])C([H])([H])C(=O)N(C([H])([H])[H])c1c([H])c(c([H])c(OC([H])([H])[H])c1Cl)C([H])([H])/C(C([H])([H])[H])=C([H])/C([H])=C(\[H])[C@@]2([H])OC([H])([H])[H] |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C35H48ClN3O10S/c1-19-10-9-11-26(46-8)35(44)18-25(47-33(43)37-35)20(2)31-34(4,49-31)27(48-32(42)21(3)38(5)28(40)12-13-50)17-29(41)39(6)23-15-22(14-19)16-24(45-7)30(23)36/h9-11,15-16,20-21,25-27,31,44,50H,12-14,17-18H2,1-8H3,(H,37,43)/b11-9+,19-10+/t20-,21+,25+,26-,27+,31+,34+,35+/m1/s1
|
|||||
| InChIKey |
ANZJBCHSOXCCRQ-FKUXLPTCSA-N
|
|||||
| IUPAC Name |
[(1S,2R,3S,5S,6S,16E,18E,20R,21S)-11-chloro-21-hydroxy-12,20-dimethoxy-2,5,9,16-tetramethyl-8,23-dioxo-4,24-dioxa-9,22-diazatetracyclo[19.3.1.110,14.03,5]hexacosa-10,12,14(26),16,18-pentaen-6-yl] (2S)-2-[methyl(3-sulfanylpropanoyl)amino]propanoate
|
|||||
| Pharmaceutical Properties | Molecule Weight |
738.3 |
Polar area |
156.47 |
||
Complexity |
737.2748934 |
xlogp Value |
3.8344 |
|||
Heavy Count |
50 |
Rot Bonds |
17 |
|||
Hbond acc |
11 |
Hbond Donor |
3 |
|||
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
Trastuzumab emtansine [Approved]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
21.40%
|
Positive HER2 expression (HER2 +++/++) | ||
| Patients Enrolled |
Untreated, asymptomatic BM or controlled brain disease treated with radiotherapy >14 days before enrollment; had received prior HER2-targeted therapy and chemotherapy; and had progressed on or after their most recent treatment of advanced breast cancer.
|
||||
| Administration Dosage |
3.6 mg/kg intravenously every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01702571 | Phase Status | Phase 3 | ||
| Clinical Description |
A two-cohort, open-label, multicenter study of trastuzumab emtansine (T-DM1) in HER2-positive locally advanced or metastatic breast cancer patients who have received prior anti-HER2 and chemotherapy-based treatment.
|
||||
| Primary Endpoint |
Objective response rate=21.40% (95% CI 14.60-29.60), clinical benefit rate=42.90% (95% CI 34.10-52.00).
|
||||
| Other Endpoint |
Median PFS=5.50 months (95% CI, 5.30-5.60), overall survival =18.90 months (95% CI, 17.10-21.30).
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
5.10%
|
Positive HER2 expression (HER2+++/++) | ||
| Patients Enrolled |
HER2 positive advanced urothelial bladder cancer or pancreatic cancer/cholangiocarcinoma
|
||||
| Administration Dosage |
Received singleagent TDM1 2.4 mg/kg once weekly (qw) or 3.6 mg/kg every 3 weeks (q3w).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02999672 | Phase Status | Phase 2 | ||
| Clinical Description |
A study to determine best tumor response with trastuzumab emtansine in human epidermal growth factor receptor 2 (HER2) overexpressing solid tumors (KAMELEON).
|
||||
| Primary Endpoint |
BOR, Urothelial bladder cancer (n=13); PR N=5 (38.46%);SD N=1 (7.7%);PD N=6 (46.2%);NE N=1 (7.69%). Pancreatic cancer/cholangiocarcinoma (n=7) PR N=1 (14.29%);SD N=3 (42.86%);PD N=2 (28.57%); NE N=1 (14.29%).
|
||||
| Other Endpoint |
PFS, Urothelial bladder cancer (n=13), Median PFS, months (95% CI) 2.20 (1.18-4.30), Median OS, months (95% CI) 7.03 (3.75-NE). Pancreatic cancer/cholangiocarcinoma (n=7), Median PFS, months (95% CI) 2.58 (1.31-9.99), Median OS, months (95% CI) NE (1.45-NE).
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
5.60% (Day 21)
|
|||
| Patients Enrolled |
38 patients were enrolled and 36 included in efficacy analysis.Patients were treated with the standard intravenous dosing of T- DM1, that is 3.6mg/kg every 3 weeks for a 21-day cycle.
|
||||
| Administration Dosage |
3.6 mg/kg every 3 weeks for a 21-day cycle, until toxicity or progression.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02465060 | Phase Status | Phase 2 | ||
| Clinical Description |
Targeted therapy directed by genetic testing in treating patients with advanced refractory solid tumors, lymphomas, or multiple myeloma (the MATCH screening trial).
|
||||
| Primary Endpoint |
ORR was 2/36 (5.56%) with 90% confidence interval (90% CI, 1.00% to 16.50%)
|
||||
| Other Endpoint |
6-mouth sPFS=23.60%.
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
45.00%
|
Positive HER2 expression (HER2 +++/++) | ||
| Patients Enrolled |
An Eastern Cooperative Oncology Group performance status of 0 or 1 and centrally confirmed, measurable, HER2-positive advanced breast cancer previously treated with trastuzumab and a taxane.
|
||||
| Administration Dosage |
3.6 mg/kg ( trastuzumab emtansine) and 1200 mg (Atezolizumab) intravenously every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02924883 | Phase Status | Phase 2 | ||
| Clinical Description |
A randomized, multicenter, double-blind, placebo-controlled phase II study of the efficacy and safety of trastuzumab emtansine in combination with atezolizumab or atezolizumab-placebo in patients with HER2-positive locally advanced or metastatic breast cancer who have received prior trastuzumab and taxane based therapy.
|
||||
| Primary Endpoint |
Median PFS=8.20 months (95% CI 5.80-10.70).
|
||||
| Other Endpoint |
Median overall survival was not estimable (95% CI NE-NE); Objective response rate=45.00% (95% CI 28.06-50.30).
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
20.00%
|
High HER2 expression (HER2+++) | ||
| Patients Enrolled |
HER2-positive, metastatic breast cancer previously treated with taxane, trastuzumab, and pertuzumab, and were T-DM1-nave.
|
||||
| Administration Dosage |
The study consisted of a dose de-escalation (dose-finding) cohort, followed by an expansion cohort at the recommended phase II dose (RP2D), T-DM1 3.60 mg/kg intravenously every 21 days, and pembrolizumab 200mg intravenously every 21 days, if one or fewer DLTs were noted in the first six patients at that dose level, it would be declared the RP2D.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03032107 | Phase Status | Phase 1b | ||
| Clinical Description |
A phase 1b study of pembrolizumab in combination with trastuzumab-dm1 in metastatic HER2-positive breast cancer.
|
||||
| Primary Endpoint |
OrR=20.00% (95% CI 5.70%-43.70%), and median PFS=9.60 months (95%CI 2.80-16.00 months).
|
||||
| Other Endpoint |
There were no dose-limiting toxicities. The RP2D was 3.60 mg/kg T-DM1 plus 200 mg pembrolizumab every 21 days.
|
||||
| Experiment 6 Reporting the Activity Date of This ADC | [6] | ||||
| Patients Enrolled |
Newly diagnosed, HER2-positive, nonmetastatic, histologically confirmed, operable primary invasive breast carcinoma.
|
||||
| Administration Dosage |
T-DM1 was dosed at 3.60 mg/kg once every 3 weeks. Trastuzumab was dosed at 6 mg/kg once every 3 weeks after an 8 mg/kg loading dose and started concurrently with the taxane. Pertuzumab was dosed at 420 mg once every 3 weeks after an 840 mg loading dose and administered concurrently with T-DM1 or trastuzumab-plus-taxane. An interval of 3 weeks from the last dose of anthracycline to initiation of HER2-targeted therapy was required. After the taxane-concurrent phase in the trastuzumab-containing arm, trastuzumab-plus-pertuzumab was continued for 1 year. In the T-DM1containing arm, T-DM1-plus-pertuzumab was continued for 1 year.
Click to Show/Hide
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01966471 | Phase Status | Phase 3 | ||
| Clinical Description |
A randomized, multicenter, open-label, phase 3 trial comparing trastuzumab plus pertuzumab plus a taxane following anthracyclines versus trastuzumab emtansine plus pertuzumab following anthracyclines as adjuvant therapy in patients with operable HER2-positive primary breast cancer.
|
||||
| Primary Endpoint |
82 (9.90%) IDFS events had occurred in the AC-THP arm and 80 (9.60%) had occurred in the AC-KP arm.
|
||||
| Other Endpoint |
3-year IDFS rates were 94.10% (95% CI, 92.50 to 95.70) with AC-THP and 92.80% (95% CI, 91.00 to 94.50) with AC-KP.
|
||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 1.10% (Day 28) | Low HER2 expression (HER2+) | ||
| Method Description |
The antitumor activity of T-DM1 was evaluated in various patient-derived xenograft models with different HER2 expression levels. Each cell suspension or tumor fragment was inoculated subcutaneously into specific pathogen-free female nude mice.The tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was initiated on day 0. In this group, 10 mg/kg T-DM1 was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: ST565) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 19.20% (Day 28) | Low HER2 expression (HER2+) | ||
| Method Description |
The antitumor activity of T-DM1 was evaluated in various patient-derived xenograft models with different HER2 expression levels. Each cell suspension or tumor fragment was inoculated subcutaneously into specific pathogen-free female nude mice.The tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was initiated on day 0. In this group, 10 mg/kg T-DM1 was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: ST313) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 37.60% (Day 28) | High HER2 expression (HER2+++) | ||
| Method Description |
The antitumor activity of T-DM1 was evaluated in various patient-derived xenograft models with different HER2 expression levels. Each cell suspension or tumor fragment was inoculated subcutaneously into specific pathogen-free female nude mice.The tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was initiated on day 0. In this group, 10 mg/kg T-DM1 was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX model: NIBIO G016) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 85.20% (Day 21) | Moderate HER2 expression (HER2++) | ||
| Method Description |
The antitumor activity of T-DM1 was evaluated in various patient-derived xenograft models with different HER2 expression levels. Each cell suspension or tumor fragment was inoculated subcutaneously into specific pathogen-free female nude mice.The tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was initiated on day 0. In this group, 10 mg/kg T-DM1 was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: ST225) | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.40% | Positive HER2 expression (HER2+++/++) | ||
| Method Description |
T-DM1 (Genentech) was administered i.p. at 10 mg/kg once per week.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: PDX12) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 7.10% (Day 21) | Low HER2 expression (HER2+) | ||
| Method Description |
The antitumor activity of T-DM1 was evaluated in various mice xenograft models with different HER2 expression levels; CFPAC-1 (low-expression). Each cell suspension or tumor fragment was inoculated subcutaneously into specific pathogen-free female nude mice.The tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was initiated on day 0. In this group, 10 mg/kg T-DM1 was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | CFPAC-1 cell line xenograft model | ||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | CFPAC-1 cells | CVCL_1119 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 15.50% (Day 7) | High HER2 expression (HER2+++) | ||
| Method Description |
The activity of trastuzumab-MCC-DM1 was further investigated in trastuzumab-insensitive mouse xenograft model. After transplantation of MMTV-HER2 Fo5 mammary tumor explants, tumor-bearing nude mice (n = 8 mice per group) were treated once every 3 weeks with 1 mg/kg trastuzmab-MCC-DM1.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
| Experiment 3 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 27.10% (Day 48) | High HER2 expression (HER2+++) | ||
| Method Description |
Naive female beige nude XID mice were inoculated in the mammary fat pad with 20 million tumor cells suspended in 50% phenol redfree Matrigel mixed with culture medium. All animals were randomly assigned into treatment groups, such that the mean tumor volume for each group was 100 to 200 mm3. Nude mice with established tumors were dosed i.v. with Tmab-MCC-DM1 0.3 mg/kg for a total of three injections.
Click to Show/Hide
|
||||
| In Vivo Model | Trastuzumab-resistant breast cancer CDX model | ||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-474 EEI cells | CVCL_AR96 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 31.70% (Day 21) | Low HER2 expression (HER2+) | ||
| Method Description |
The antitumor activity of T-DM1 was evaluated in various mice xenograft models with different HER2 expression levels; Capan-1 (weak positive). Each cell suspension or tumor fragment was inoculated subcutaneously into specific pathogen-free female nude mice.The tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was initiated on day 0. In this group, 10 mg/kg T-DM1 was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Capan-1 cell line xenograft model | ||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | Capan-1 cells | CVCL_0237 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 33.10% (Day 21) | Negative HER2 expression (HER2-) | ||
| Method Description |
The antitumor activity of T-DM1 was evaluated in various mice xenograft models with different HER2 expression levels; GCIY (negative). Each cell suspension or tumor fragment was inoculated subcutaneously into specific pathogen-free female nude mice.The tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was initiated on day 0. In this group, 10 mg/kg T-DM1 was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | GCIY cell line xenograft model | ||||
| In Vitro Model | Gastric adenocarcinoma | GCIY cells | CVCL_1228 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 36.00% (Day 33) | Moderate HER2 expression (HER2++) | ||
| Method Description |
Subcutaneous injection of 100,000 cells mixed in 200 mL PBS solution was performed. Two weeks after tumour cell injection, mice were treated with trastuzumab, cetuximab or T-DM1 at a dose of 300 mg/kg via IP injection.
|
||||
| In Vivo Model | HT29 CDX model | ||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 7 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 36.00% (Day 33) | Low HER2 expression (HER2 +) | ||
| Method Description |
SW48, HT-29 and LS174T cells were injected into null mice subcutaneously, followed by treatment with cetuximab, trastuzumab and T-DM1. Two weeks after tumour cell injection, mice were treated with trastuzumab, cetuximab or T-DM1 at a dose of 300 mg/kg via IP injection.
|
||||
| In Vivo Model | SW48 CDX model | ||||
| In Vitro Model | Colon adenocarcinoma | SW48 cells | CVCL_1724 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 42.00% (Day 58) | Low HER2 expression (HER2 +) | ||
| Method Description |
11-18 cells (4,000,000 ) and HCC827 cells (2,000,000 ) were injected subcutaneously into the backs on both sides of the mice. T-DM1 (30 mg/kg, once per week i.p.) for 8 weeks.
|
||||
| In Vivo Model | 11-18 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | 11-18 cells | CVCL_6659 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 42.00% (Day 58) | Low HER2 expression (HER2+) | ||
| Method Description |
Subcutaneous injection of 100,000 cells mixed in 200 mL PBS solution was performed. Two weeks after tumour cell injection, mice were treated with trastuzumab, cetuximab or T-DM1 (30 mg/kg, once per week i.p.) for 8 weeks.
|
||||
| In Vivo Model | 11-18 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | 11-18 cells | CVCL_6659 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 43.30% (Day 30) | Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
Trastuzumab-emtansine (3 mg/kg, every seven days 4) induces efficient tumor cell killing in cell line-derived models of BT-474/R1-7 cells with HER2 expression with high expression.
|
||||
| In Vivo Model | BT-474 CDX model (T-DM1 resistant) | ||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells (Trastuzumab emtansine resistant) | CVCL_0179 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.00% (Day 21) | Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
Trastuzumab-emtansine (3 mg/kg, every seven days x3) induces efficient tumor cell killing in cell line-derived models of SK-OV-3 cells with HER2 expression with high expression.
|
||||
| In Vivo Model | SK-OV-3 CDX model (Expressing YES1 Y537F) | ||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells (YES1 Y537F expression) | CVCL_0532 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.50% (Day 48) | High HER2 expression (HER2+++) | ||
| Method Description |
Naive female beige nude XID mice were inoculated in the mammary fat pad with 20 million tumor cells suspended in 50% phenol redfree Matrigel mixed with culture medium. All animals were randomly assigned into treatment groups, such that the mean tumor volume for each group was 100 to 200 mm3. Nude mice with established tumors were dosed i.v. with Tmab-MCC-DM1 1 mg/kg for a total of three injections.
Click to Show/Hide
|
||||
| In Vivo Model | Trastuzumab-resistant breast cancer CDX model | ||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-474 EEI cells | CVCL_AR96 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 46.20% (Day 7) | High HER2 expression (HER2+++) | ||
| Method Description |
The activity of trastuzumab-MCC-DM1 was further investigated in trastuzumab-insensitive mouse xenograft model. After transplantation of MMTV-HER2 Fo5 mammary tumor explants, tumor-bearing nude mice (n = 8 mice per group) were treated once every 3 weeks with 3 mg/kg trastuzmab-MCC-DM1.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
| Experiment 14 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 55.00% (Day 33) | Positive HER2 expression (HER2+++/++) | ||
| Method Description |
Subcutaneous injection of 100,000 cells mixed in 200 mL PBS solution was performed. Two weeks after tumour cell injection, mice were treated with trastuzumab, cetuximab or T-DM1 at a dose of 300 mg/kg via IP injection.
|
||||
| In Vivo Model | HT-29 CDX model | ||||
| In Vitro Model | Colon adenocarcinoma | HT-29 cells | CVCL_0320 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 55.00% (Day 33) | Moderate HER2 expression (HER2 ++) | ||
| Method Description |
SW48, HT-29 and LS174T cells were injected into null mice subcutaneously, followed by treatment with cetuximab, trastuzumab and T-DM1. Two weeks after tumour cell injection, mice were treated with trastuzumab, cetuximab or T-DM1 at a dose of 300 mg/kg via IP injection.
|
||||
| In Vivo Model | HT-29 CDX model | ||||
| In Vitro Model | Colon adenocarcinoma | HT-29 cells | CVCL_0320 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 58.50% (Day 21) | Moderate HER2 expression (HER2++) | ||
| Method Description |
The antitumor activity of T-DM1 was evaluated in various mice xenograft models with different HER2 expression levels; JIMT-1 (moderate positive). Each cell suspension or tumor fragment was inoculated subcutaneously into specific pathogen-free female nude mice.The tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was initiated on day 0. In this group, 10 mg/kg T-DM1 was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | JIMT-1 cell line xenograft model | ||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 58.70% (Day 48) | High HER2 expression (HER2+++) | ||
| Method Description |
Naive female beige nude XID mice were inoculated in the mammary fat pad with 20 million tumor cells suspended in 50% phenol redfree Matrigel mixed with culture medium. All animals were randomly assigned into treatment groups, such that the mean tumor volume for each group was 100 to 200 mm3. Nude mice with established tumors were dosed i.v. with Tmab-MCC-DM1 3 mg/kg for a total of three injections.
Click to Show/Hide
|
||||
| In Vivo Model | Trastuzumab-resistant breast cancer CDX model | ||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-474 EEI cells | CVCL_AR96 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 63.00% (Day 58) | Positive HER2 expression (HER2+++/++) | ||
| Method Description |
Subcutaneous injection of 100,000 cells mixed in 200 mL PBS solution was performed. Two weeks after tumour cell injection, mice were treated with trastuzumab, cetuximab or T-DM1 (30 mg/kg, once per week i.p.) for 8 weeks.
|
||||
| In Vivo Model | H3255 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | NCI-H3255 cells | CVCL_6831 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 63.00% (Day 58) | High HER2 expression (HER2 +++) | ||
| Method Description |
T-DM1 (30 mg/kg, once per week i.p.) for 8 weeks,.
|
||||
| In Vivo Model | H3255 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | NCI-H3255 cells | CVCL_6831 | ||
| Experiment 20 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 63.30% (Day 33) | High HER2 expression (HER2 +++) | ||
| Method Description |
SW48, HT-29 and LS174T cells were injected into null mice subcutaneously, followed by treatment with cetuximab, trastuzumab and T-DM1. Two weeks after tumour cell injection, mice were treated with trastuzumab, cetuximab or T-DM1 at a dose of 300 mg/kg via IP injection.
|
||||
| In Vivo Model | LS174T CDX model | ||||
| In Vitro Model | Colon adenocarcinoma | LS174T cells | CVCL_1384 | ||
| Experiment 21 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 66.70% (Day 51) | Low HER2 expression (HER2+) | ||
| Method Description |
Subcutaneous injection of 100,000 cells mixed in 200 mL PBS solution was performed. Two weeks after tumour cell injection, mice were treated with trastuzumab, cetuximab or T-DM1 (30 mg/kg, once per week i.p.) for 8 weeks.
|
||||
| In Vivo Model | HCC827 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | HCC827 cells | CVCL_2063 | ||
| Experiment 22 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 74.00% (Day 7) | High HER2 expression (HER2+++) | ||
| Method Description |
The activity of trastuzumab-MCC-DM1 was further investigated in trastuzumab-insensitive mouse xenograft model. After transplantation of MMTV-HER2 Fo5 mammary tumor explants, tumor-bearing nude mice (n = 8 mice per group) were treated once every 3 weeks with 10 mg/kg trastuzmab-MCC-DM1.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
| Experiment 23 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 76.54% (Day 24) | Positive HER2 expression (HER2+++/++) | ||
| Method Description |
Inoculate 150 mice with KPL-4 cells at 3 million cells/mouse suspended in HBSS/matrigel, in the thoracic mammary fat pad at a volume of 0.2 ml. When tumors have reached a mean tumor volume of 100-250 mm3, they will be grouped out into 10 groups of 8-10 mice each. A single treatment will be administered intravenously (1 mg/kg) via the tail vein on Day 0.
Click to Show/Hide
|
||||
| In Vivo Model | KPL-4 CDX model | ||||
| In Vitro Model | Breast inflammatory carcinoma | KPL-4 cells | CVCL_5310 | ||
| Experiment 24 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 82.90% (Day 24) | Positive HER2 expression (HER2+++/++) | ||
| Method Description |
Inoculate 150 mice with KPL-4 cells at 3 million cells/mouse suspended in HBSS/matrigel, in the thoracic mammary fat pad at a volume of 0.2 ml. When tumors have reached a mean tumor volume of 100-250 mm3, they will be grouped out into 10 groups of 8-10 mice each. A single treatment will be administered intravenously (ADC-211, 1 mg/kg plus ADC-106, 5 mg/kg) via the tail vein on Day 0.
Click to Show/Hide
|
||||
| In Vivo Model | KPL-4 CDX model | ||||
| In Vitro Model | Breast inflammatory carcinoma | KPL-4 cells | CVCL_5310 | ||
| Experiment 25 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 83.90% (Day 10) | High HER2 expression (HER2+++) | ||
| Method Description |
Mice bearing mammary tumor transplants from the MMTV-HER2 Fo5 line were given a single iv injection (10 mg/kg) of Tmab-SPP-DM1, Tmab-SSNPP-DM3, Tmab-SSNPP-DM4, Tmab-MCC-DM1, or vehicle (n=7 mice per group), and tumor growth was monitored for 25 days.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
| Experiment 26 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 84.39% (Day 24) | Positive HER2 expression (HER2+++/++) | ||
| Method Description |
Inoculate 150 mice with KPL-4 cells at 3 million cells/mouse suspended in HBSS/matrigel, in the thoracic mammary fat pad at a volume of 0.2 ml. When tumors have reached a mean tumor volume of 100-250 mm3, they will be grouped out into 10 groups of 8-10 mice each. A single treatment will be administered intravenously (ADC-211, 1 mg/kg plus ADC-106, 1 mg/kg) via the tail vein on Day 0.
Click to Show/Hide
|
||||
| In Vivo Model | KPL-4 CDX model | ||||
| In Vitro Model | Breast inflammatory carcinoma | KPL-4 cells | CVCL_5310 | ||
| Experiment 27 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.70% (Day 7) | High HER2 expression (HER2+++) | ||
| Method Description |
The activity of trastuzumab-MCC-DM1 was further investigated in trastuzumab-insensitive mouse xenograft model. After transplantation of MMTV-HER2 Fo5 mammary tumor explants, tumor-bearing nude mice (n = 8 mice per group) were treated once every 3 weeks with 15 mg/kg trastuzmab-MCC-DM1.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
| Experiment 28 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.10% (Day 7) | High HER2 expression (HER2+++) | ||
| Method Description |
The activity of trastuzumab-MCC-DM1 was further investigated in trastuzumab-insensitive mouse xenograft model. After transplantation of MMTV-HER2 Fo5 mammary tumor explants, tumor-bearing nude mice (n = 8 mice per group) were treated once every 3 weeks with 30 mg/kg trastuzmab-MCC-DM1.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
| Experiment 29 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.70% (Day 48) | High HER2 expression (HER2+++) | ||
| Method Description |
Naive female beige nude XID mice were inoculated in the mammary fat pad with 20 million tumor cells suspended in 50% phenol redfree Matrigel mixed with culture medium. All animals were randomly assigned into treatment groups, such that the mean tumor volume for each group was 100 to 200 mm3. Nude mice with established tumors were dosed i.v. with Tmab-MCC-DM1 10 mg/kg for a total of three injections.
Click to Show/Hide
|
||||
| In Vivo Model | Trastuzumab-resistant breast cancer CDX model | ||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-474 EEI cells | CVCL_AR96 | ||
| Experiment 30 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 92.30% (Day 14) | High HER2 expression (HER2+++) | ||
| Method Description |
KPL-4 human breast tumor cells were inoculated (3 million cells per mouse, in Matrigel) into the mammary fat pads of SCID beige mice. All animals were randomly assigned into treatment groups, such that the mean tumor volume for each group was 100 to 200 mm3. Trastuzumab-maytansinoid conjugates were given by single 15 mg/kg iv injection.
|
||||
| In Vivo Model | Breast cancer CDX model | ||||
| In Vitro Model | Breast inflammatory carcinoma | KPL-4 cells | CVCL_5310 | ||
| Experiment 31 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.70% (Day 48) | High HER2 expression (HER2+++) | ||
| Method Description |
Naive female beige nude XID mice were inoculated in the mammary fat pad with 20 million tumor cells suspended in 50% phenol redfree Matrigel mixed with culture medium. All animals were randomly assigned into treatment groups, such that the mean tumor volume for each group was 100 to 200 mm3. Nude mice with established tumors were dosed i.v. with Tmab-MCC-DM1 15 mg/kg for a total of three injections.
Click to Show/Hide
|
||||
| In Vivo Model | Trastuzumab-resistant breast cancer CDX model | ||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-474 EEI cells | CVCL_AR96 | ||
| Experiment 32 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.40% (Day 21) | High HER2 expression (HER2+++) | ||
| Method Description |
The antitumor activity of T-DM1 was evaluated in various mice xenograft models with different HER2 expression levels; KPL-4 (strong positive). Each cell suspension or tumor fragment was inoculated subcutaneously into specific pathogen-free female nude mice.The tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was initiated on day 0. In this group, 10 mg/kg T-DM1 was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | KPL-4 cell line xenograft model | ||||
| In Vitro Model | Breast inflammatory carcinoma | KPL-4 cells | CVCL_5310 | ||
| Experiment 33 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 56) | Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
HER2-positive HCC1954 cells were either treated with vehicle (control) or T-DM1 for 5 days, trypsinized, washed in PBS and sorted by flow cytometry based on the surface ROR1 expression. ROR1- and ROR1+ or unfractionated cells were injected into the subcutaneous site of 6-8-week old Nu/J mice (n=3/group).
|
||||
| In Vivo Model | HCC1954 CDX model | ||||
| In Vitro Model | Breast ductal carcinoma | HCC1954 cells | CVCL_1259 | ||
| Experiment 34 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 35) | High HER2 expression (HER2+++) | ||
| Method Description |
Mice were inoculated subcutaneously in the right flank with either 5x106 cells/mouse of BTC cell line KKU-100, mice were randomized to the control group or treatment with T-DM1 20 mg/kg groups.
|
||||
| In Vivo Model | KMCH-1 CDX model | ||||
| In Vitro Model | Combined hepatocellular carcinoma and cholangiocarcinoma | KMCH-1 cells | CVCL_7970 | ||
| Experiment 35 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Minimal Effective Dose (MED) | < 5.00 mg/kg | Moderate HER2 expression (HER2++) | ||
| Method Description |
Following the acclimatization period (1 week), the animals were stratified by body weight and randomly assigned to the following group: two T-DM1 groups, treated with 20 mg/kg and 60 mg/kg. Each group consisted of five animals for blood chemistry test as well as five animals for clinical signs and body weight measurements.
|
||||
| In Vivo Model | Gastric cancer CDX model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 36 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Maximum Tolerated Dose (MTD) | > 20.00 mg/kg | High HER2 expression (HER2+++) | ||
| Method Description |
Following the acclimatization period (1 week), the animals were stratified by body weight and randomly assigned to the following group: two T-DM1 groups, treated with 20 mg/kg and 60 mg/kg. Each group consisted of five animals for blood chemistry test as well as five animals for clinical signs and body weight measurements.
|
||||
| In Vivo Model | Gastric cancer CDX model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
Obtained from the Model Organism Data
| Experiment 1 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.98% (Day 7) | High HER2 expression (HER2 +++) | ||
| Method Description |
An allograft was propagated from the Fo5 mmty transgenic mouse which does not respond to.or responds poorly to HERCEPTIN therapy. Subjects were treated once with ADC (10 mg/kg); and placebo PBS buffer control (Vehicle) andmonitored over 3 weeks.
|
||||
| In Vivo Model | Breast cancer model MMTV-HER2 Fo5 | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [18] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [18] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.14 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.24 nM±0.12 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [18] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.26 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.57 nM±0.10 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [18] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.76 nM
|
Moderate HER2 expression (HER2 ++) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.71 nM
|
|||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. For measurement of apoptosis, BT-474 and SK-BR-3 were exposed to trastuzumab or trastuzumab-DM for 48 h.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.26 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. For measurement of apoptosis, BT-474 and SK-BR-3 were exposed to trastuzumab or trastuzumab-DM for 48 h.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.89 nM±3.51 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
6.40 nM±4.80 nM
|
Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
ADCs 21-23 was performed on HCC827 and NCI-H2228 cells. cells (5 x 103 cells/well) were cultured in 96-well plates with 100 uL complete medium, and 24 h later the cells were treated in triplicate with varying concentrations of ADCs for 72 h.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
18.37 nM
|
Low HER2 expression (HER2+; IHC 1+) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [18] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 50.00 nM | Negative HER2 expression (HER2 -) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100.00 nM | Negative HER2 expression (HER2 -) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
220.00 nM±39.50 nM
|
Negative HER2 expression (HER2 -) | ||
| Method Description |
ADCs 21-23 was performed on HCC827 and NCI-H2228 cells. cells (5 x 103 cells/well) were cultured in 96-well plates with 100 uL complete medium, and 24 h later the cells were treated in triplicate with varying concentrations of ADCs for 72 h.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
317.00 nM±93.70 nM
|
Negative HER2 expression (HER2 -) | ||
| Method Description |
ADCs 21-23 was performed on HCC827 and NCI-H2228 cells. cells (5 x 103 cells/well) were cultured in 96-well plates with 100 uL complete medium, and 24 h later the cells were treated in triplicate with varying concentrations of ADCs for 72 h.
|
||||
| In Vitro Model | Lung adenocarcinoma | HCC827 cells | CVCL_2063 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 500 nM | Negative HER2 expression (HER2 -) | ||
| Method Description |
ADCs 21-23 was performed on HCC827 and NCI-H2228 cells. cells (5 x 103 cells/well) were cultured in 96-well plates with 100 uL complete medium, and 24 h later the cells were treated in triplicate with varying concentrations of ADCs for 72 h.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
8.00-15.00 ng/mL
|
High HER2 expression (HER2+++) | ||
| Method Description |
Exposing mammalian cells having HER2 receptors to ADC in a cell culture medium; culturing the cells for a period from about 6 hours to about 5 days; and measuring cell viability Cell-based in vitro assays were used to measure viability, cytotoxicity, and induction of apoptosis of the ADC of the invention.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.00 ug/mL
|
Negative HER2 expression (HER2-) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-474 EEI cells | CVCL_AR96 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 ug/mL
|
Moderate HER2 expression (HER2++; IHC 2+) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Breast inflammatory carcinoma | KPL-4 cells | CVCL_5310 | ||
| Experiment 20 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 ug/mL
|
Positive HER2 expression (HER2+++/++; HER2 MFI=95.7) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 21 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.03 ug/mL
|
High HER2 expression (HER2+++) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Breast ductal carcinoma | HCC1954 cells | CVCL_1259 | ||
| Experiment 22 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.06 ug/mL
|
High HER2 expression (HER2+++) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | Calu-3 cells | CVCL_0609 | ||
| Experiment 23 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.27 ug/mL
|
Low HER2 expression (HER2+) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | MKN7 cells | CVCL_1417 | ||
Naratuximab emtansine [Phase 2 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
12.82%
|
Positive CD38 expression (CD38+++/++) | ||
| Patients Enrolled |
Lymphoma limited to diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), or marginal zone lymphoma (MZL). Patients were also required to have received at least one prior anti-CD20 based therapeutic regimen, have a life expectancy of greater than 3 months, an Eastern Cooperative Oncology Group Performance status of 2 or lower, and adequate hematological, renal, and hepatic function.
Click to Show/Hide
|
||||
| Administration Dosage |
Conventional 3+3 dose-escalation design; intravenously once every 3 weeks; from 0.10 to 1.80 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01534715 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1, multi-center, open-label study of IMGN529 administered intravenously in adult patients with relapsed or refractory non-Hodgkin lymphoma and chronic lymphocytic leukemia.
|
||||
| Primary Endpoint |
A total of five objective responses were observed, resulting in an overall response rate (ORR) of 12.82% (N=39). Four of these (1 complete response [CR] and 3 partial responses [PRs]) occurred in patients with DLBCL, for an ORR of 22% in this lymphoma subset.
|
||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [23] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 14.50% (Day 38) | Positive CD38 expression (CD38+++/++) | ||
| Method Description |
IMGN529 induces efficient tumor cell killing in cell line-derived models B-cell NHL of cells with lower CD27 expression, dosed at 2.5 ug/kg , 2 qw3.
|
||||
| In Vivo Model | CD37-positive NHL and CLL model | ||||
| In Vitro Model | Non-Hodgkin lymphoma | Non-Hodgkin lymphoma cells | Homo sapiens | ||
| Chronic lymphocytic leukemia | Chronic lymphocytic leukemia cells | Homo sapiens | |||
| Experiment 2 Reporting the Activity Date of This ADC | [23] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.00% (Day 38) | Positive CD38 expression (CD38+++/++) | ||
| Method Description |
IMGN529 induces efficient tumor cell killing in cell line-derived models B-cell NHL of cells with lower CD27 expression, dosed at 5 ug/kg , 2 qw3.
|
||||
| In Vivo Model | CD37-positive NHL and CLL model | ||||
| In Vitro Model | Non-Hodgkin lymphoma | Non-Hodgkin lymphoma cells | Homo sapiens | ||
| Chronic lymphocytic leukemia | Chronic lymphocytic leukemia cells | Homo sapiens | |||
| Experiment 3 Reporting the Activity Date of This ADC | [23] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 38) | Positive CD38 expression (CD38+++/++) | ||
| Method Description |
IMGN529 induces efficient tumor cell killing in cell line-derived models B-cell NHL of cells with lower CD27 expression, dosed at 10 ug/kg , 2 qw3.
|
||||
| In Vivo Model | CD37-positive NHL and CLL model | ||||
| In Vitro Model | Non-Hodgkin lymphoma | Non-Hodgkin lymphoma cells | Homo sapiens | ||
| Chronic lymphocytic leukemia | Chronic lymphocytic leukemia cells | Homo sapiens | |||
| Experiment 4 Reporting the Activity Date of This ADC | [23] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (10 mg/kg)
|
Positive CD38 expression (CD38+++/++) | ||
| Method Description |
The inhibitory activity of SGN-LIV1A against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 1 day.
|
||||
| In Vivo Model | DoHH2 CDX model | ||||
| In Vitro Model | Diffuse large B-cell lymphoma | WSU-NHL cells | CVCL_1793 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [23] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.10 nM | |||
| Method Description |
In vitro cytotoxicity was measured by incubating 5000 target cells with indicated agents in complete RPMI-1640 media for 5 days at 37°C. The viability of remaining cells was determined by a colorimetric WST-10 assay.
|
||||
| In Vivo Model | DoHH2 CDX model | ||||
| In Vitro Model | Diffuse large B-cell lymphoma germinal center B-cell type | DoHH2 cells | CVCL_1179 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [23] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.10 nM | |||
| Method Description |
In vitro cytotoxicity was measured by incubating 5000 target cells with indicated agents in complete RPMI-1640 media for 5 days at 37°C. The viability of remaining cells was determined by a colorimetric WST-11 assay.
|
||||
| In Vivo Model | DoHH2 CDX model | ||||
| In Vitro Model | Mantle cell lymphoma | Granta-519 cells | CVCL_1818 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [23] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.10 nM | |||
| Method Description |
In vitro cytotoxicity was measured by incubating 5000 target cells with indicated agents in complete RPMI-1640 media for 5 days at 37°C. The viability of remaining cells was determined by a colorimetric WST-8 assay.
|
||||
| In Vivo Model | DoHH2 CDX model | ||||
| In Vitro Model | Burkitt lymphoma | BJAB cells | CVCL_5711 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [23] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.10 nM | Positive CD38 expression (CD38+++/++) | ||
| Method Description |
In vitro cytotoxicity was measured by incubating 5000 target cells with indicated agents in complete RPMI-1640 media for 5 days at 37°C. The viability of remaining cells was determined by a colorimetric WST-9 assay.
|
||||
| In Vivo Model | DoHH2 CDX model | ||||
| In Vitro Model | Diffuse large B-cell lymphoma | Farage cells | CVCL_3302 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [23] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 1.00 nM | |||
| Method Description |
In vitro cytotoxicity was measured by incubating 5000 target cells with indicated agents in complete RPMI-1640 media for 5 days at 37°C. The viability of remaining cells was determined by a colorimetric WST-12 assay.
|
||||
| In Vivo Model | DoHH2 CDX model | ||||
| In Vitro Model | Mantle cell lymphoma | JVM-2 cells | CVCL_1319 | ||
Lorvotuzumab mertansine [Phase 2 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [22] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
28.30%
|
Positive CD56 expression (CD56+++/++) | ||
| Patients Enrolled |
Relapsed and/or Refractory CD-56-positive Multiple Myeloma.
|
||||
| Administration Dosage |
40 mg/m2 (up to a maximum of 140 mg) intravenously once every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT00346255 | Phase Status | Phase 1 | ||
| Clinical Description |
BB-10901 in treating patients with relapsed and/or refractory multiple myeloma (IMGN901).
|
||||
| Primary Endpoint |
Objective response rate=28.30%.
|
||||
| Other Endpoint |
Median progression-free survival=26.10 months (95% CI 1-89 weeks).
|
||||
AVID-100 [Phase 1/2 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [24] | ||||
| Patients Enrolled |
Patients with advanced or metastatic epithelial malignancies.
|
||||
| Administration Dosage |
20, 40, 80, 120, 180, and 220 mg/m2 every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03094169 | Phase Status | Phase 1/2 | ||
| Clinical Description |
Phase 1a/2a dose escalation trial to determine safety, tolerance, MTD, and preliminary antineoplastic activity of AVID100, in patients with advanced or metastatic solid tumors of epithelial origin.
|
||||
GQ-1001 [Phase 1]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [25] | ||||
| Efficacy Data | Partial Response (PR) |
40.00%
|
|||
| Patients Enrolled |
HER2-positive advanced solid tumors.
|
||||
| Administration Dosage |
Administered intravenously as a monotherapy on Day 1 of 21-day cycles. The starting dose was 1.20 mg/kg, followed by 2.40, 3.60, 4.80, 6.00, 7.20 and 8.40 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT04450732 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1, first-in-human, multicenter, open-label, study of GQ1001, a HER2 targeted antibody-drug conjugate, administered intravenously, in adult patients with HER2-positive advanced solid tumors.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [26] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05575804 | Phase Status | Phase 1/2 | ||
| Clinical Description |
Phase 1b/2 study of GQ1001 and pyrotinib in HER2 positive metastatic breast cancer patients who had failed previous anti-HER2 treatment GRACE.
|
||||
B-003 [Phase 1]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [27] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03953833 | Phase Status | Phase 1 | ||
| Clinical Description |
Phase 1 clinical trial on the safety, tolerability, pharmacokinetics of B003 in the treatment of HER2-positive recurrent or metastatic breast cancer.
|
||||
Bivatuzumab mertansine [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [28] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
0.00%
|
|||
| Patients Enrolled |
Metastatic breast cancer (MBC) that expresses CD44v6 in at least 50% of tumor cells in primary tumor tissue as assessed by immunohistochemistry, pretreatment with anthracyclines and taxanes, tumor metastases measurable by computed tomography (CT) or magnetic resonance imaging (MRI), life expectancy of at least 6 months, no chemotherapy, radiotherapy or immunotherapy within the last 4 weeks before study entry, adequate organ function, Eastern Cooperative Oncology Group performance score 2.
Click to Show/Hide
|
||||
| Administration Dosage |
One single intravenous infusion over 30 min, dose was escalated in 25 mg/m2 increments up to the maximum tolerated dose (MTD).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02254005 | Phase Status | Phase 1 | ||
| Clinical Description |
An open phase 1 single dose escalation study of bivatuzumab mertansine administered intravenously in female patients with CD44v6 positive metastatic breast cancer with repeated administration in patients with clinical benefit.
|
||||
| Primary Endpoint |
The MTD in this trial could not be determined.
|
||||
| Other Endpoint |
No objective responses were observed. Disease stabilization was achieved in 50.00% of patients independently of dose level.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [33] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02254044 | Phase Status | Phase 1 | ||
| Clinical Description |
An open phase 1 dose escalation study of bivatuzumab mertansine administered intravenously once per week for three weeks in patients with advanced squamous cell carcinoma of the head and neck or esophagus with repeated administration courses in patients with clinical benefit.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [34] | ||||
| Patients Enrolled |
Ecurrent or metastatic head and neck squamous cell carcinoma (HNSCC) not amenable to established treatments, an ECOG score 2, and an estimated life expectancy of at least 6 months, a tumor diameter of at least 1 cm in CT or MRI scans was also required.
|
||||
| Administration Dosage |
Starting with 25 mg/m2, the dose was escalated in steps of 25 mg/m2 until dose limiting toxicity was observed, intravenously.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02254044 | Phase Status | Phase 1 | ||
| Clinical Description |
An open phase 1 dose escalation study of bivatuzumab mertansine administered intravenously once per week for three weeks in patients with advanced squamous cell carcinoma of the head and neck or esophagus with repeated administration courses in patients with clinical benefit.
|
||||
| Primary Endpoint |
The MTD was 300 mg/m2.
|
||||
| Other Endpoint |
Due to the premature discontinuation of the trial efficacy complying with the study plan could not be assessed. In 3 patients,a partial response at doses of 2.00, 2.75 and 3.25 mg/m2.
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [35] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02254031 | Phase Status | Phase 1 | ||
| Clinical Description |
An open phase 1 dose escalation study of bivatuzumab mertansine administered intravenously once per week for three weeks in female patients with CD44V6 positive recurrent or metastatic breast cancer with repeated administration courses in patients with clinical.
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [36] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02254018 | Phase Status | Phase 1 | ||
| Clinical Description |
An open phase 1 single dose escalation study of bivatuzumab mertansine administered intravenously in patients with advanced squamous cell carcinoma of the head and neck with repeated administration in patients with clinical benefit.
|
||||
| Experiment 6 Reporting the Activity Date of This ADC | [37] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02254005 | Phase Status | Phase 1 | ||
| Clinical Description |
An open phase 1 single dose escalation study of bivatuzumab mertansine administered intravenously in female patients with CD44v6 positive metastatic breast cancer with repeated administration in patients with clinical benefit.
|
||||
AMG-224 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [29] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
23.00% (heavily pretreated population of patients with R/R MM with a median of seven lines of prior therapy)
60.00% (in a phase 1 trial of GSK2857916) |
|||
| Patients Enrolled |
Relapsed or refractory (R/R) multiple myeloma (MM).
|
||||
| Administration Dosage |
Every 3 weeks (Q3W) at prespecified doses of 30-300 mg in a 3+3 design.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02561962 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 first in human study evaluating the safety, tolerability, pharmacokinetics and pharmacodynamics of AMG 224 in subjects with relapsed or refractory multiple myeloma.
|
||||
| Primary Endpoint |
AmG 224 was generally well tolerated up to 190 mg Q3W.
|
||||
| Other Endpoint |
ORR=23.00% (95% CI,11.00-39.00%), including six responses in dose escalation and three responses in the dose expansion. Two (5.00%) patients were CR and 7 (18.00%) patients were PR.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [38] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02561962 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 first in human study evaluating the safety, tolerability, pharmacokinetics and pharmacodynamics of AMG 224 in subjects with relapsed or refractory multiple myeloma.
|
||||
Cantuzumab mertansine [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [30] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
0.00%
|
|||
| Patients Enrolled |
Histological documentation of advanced or metastatic epithelial solid tumor which were likely to express the CanAg antigen, that were refractory or resistant to standard chemotherapy, or for which no effective standard therapy exists.
|
||||
| Administration Dosage |
IV infusion at an initial dose of 30 mg/m2, three times per week for three consecutive weeks for a total of nine doses.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [40] | ||||
| Patients Enrolled |
Solid malignancies refractory to standard therapy or for whom no standard therapy existed.
|
||||
| Administration Dosage |
IV cantuzumab mertansine was administered at a rate of 1 mg/min for 30 minutes and then increased to 3 mg/min if hypersensitivity phenomena were not observed. Treatment courses were repeated every 3 weeks.
|
||||
PCA-062 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Disease Control Rate (DCR) |
22.60% (31 patients with other tumors)
33.30% (HNSCC) 22.20% (esophageal cancer) |
|||
| Patients Enrolled |
Advanced solid tumors expressing P-cadherin, TNBC, head and neck squamous cell carcinoma (HNSCC), esophageal cancer, cervical cancer, and non-small cell lung cancer (NSCLC).
|
||||
| Administration Dosage |
At 10 different dose levels of PCA062, ranging from 0.40 to 5.00 mg/kg every 2 weeks administered as a 1-hour intravenous infusion.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02375958 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 multi-center, open-label dose escalation and expansion study of PCA062 administered intravenously in adult patients with p-CAD positive tumors.
|
||||
| Primary Endpoint |
The MTD was PCA062 3.60 mg/kg every 2 weeks.No patient achieved a complete response. Only 1 patient with stage IV metastatic HNSCC treated at 0.90 mg/kg achieved a confirmed partial response (PR) as best overall response (BOR). The disease control rate (DCR) for the 31 patients with other tumors was 22.60% (95% CI, 9.60-41.10). In patients with HNSCC (n = 6), DCR was 33.30% (95% CI, 4.30-77.70), and in patients with esophageal cancer (n = 9), DCR was 22.20% (95% CI, 2.80-60.00).
Click to Show/Hide
|
||||
Laprituximab emtansine [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [32] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT01963715 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1, multi-center, open-label study of IMGN289 administered intravenously in adult patients with EGFR-positive solid tumors.
|
||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [42] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
58.30%
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
The cytotoxic activity of IMGN289 was evaluated against a panel of SCCHN cell lines in vitro. Immunodeficient mice bearing established subcutaneous xenograft tumors were treated with a single intravenous injection of IMGN289 at 1,2.5 or 5.0 mg/kg (based on antibody concentration). IMGN289 dose was 5 mg/kg administered as a single injection.
|
||||
| In Vivo Model | Head and neck squamous cell carcinomas CDX model | ||||
| In Vitro Model | Hypopharyngeal squamous cell carcinoma | FaDu cells | CVCL_1218 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [42] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
83.30%
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
The cytotoxic activity of IMGN289 was evaluated against a panel of SCCHN cell lines in vitro. Immunodeficient mice bearing established subcutaneous xenograft tumors were treated with a single intravenous injection of IMGN289 at 1,2.5 or 5.0 mg/kg (based on antibody concentration). IMGN289 dose was 5 mg/kg administered as a single injection.
|
||||
| In Vivo Model | Head and neck squamous cell carcinomas CDX model | ||||
| In Vitro Model | Oral cavity squamous cell carcinoma | HSC-2 cells | CVCL_1287 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [42] | ||||
| Efficacy Data | Minimal Effective Dose (MED) |
1.00 mg/kg
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
The cytotoxic activity of IMGN289 was evaluated against a panel of SCCHN cell lines in vitro. Immunodeficient mice bearing established subcutaneous xenograft tumors were treated with a single intravenous injection of IMGN289 at 1,2.5 or 5.0 mg/kg (based on antibody concentration).
|
||||
| In Vivo Model | Head and neck squamous cell carcinomas CDX model | ||||
| In Vitro Model | Hypopharyngeal squamous cell carcinoma | FaDu cells | CVCL_1218 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [42] | ||||
| Efficacy Data | Minimal Effective Dose (MED) |
2.50 mg/kg
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
The cytotoxic activity of IMGN289 was evaluated against a panel of SCCHN cell lines in vitro. Immunodeficient mice bearing established subcutaneous xenograft tumors were treated with a single intravenous injection of IMGN289 at 1,2.5 or 5.0 mg/kg (based on antibody concentration).
|
||||
| In Vivo Model | Head and neck squamous cell carcinomas CDX model | ||||
| In Vitro Model | Oral cavity squamous cell carcinoma | HSC-2 cells | CVCL_1287 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [43] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.25 nM
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
Effects of IMGN289 on clonogenicity and proliferation were tested in HNSCC cell lines with varying cetuximab and gefitinib sensitivities.
|
||||
| In Vitro Model | Head and neck squamous cell carcinoma | HNSCC cells | Homo sapiens | ||
SHR-A1201 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [39] | ||||
| Patients Enrolled |
HER2-positive locally advanced or metastatic breast cancer (positivity for HER2 was defined as a score of 2+ in immunohistochemical analysis and a positive result in fluorescence in situ hybridization or a score of 3+ in immunohistochemical analysis), the standard treatment was invalid or there was no effective standard treatment plan, the Eastern Cooperative Oncology Group performance status score was 0-1.
Click to Show/Hide
|
||||
| Administration Dosage |
4 dose-escalation sequences, 1.20 mg/kg, 2.40 mg/kg, 3.60 mg/kg and 4.80 mg/kg, once every 21days, as a 90-min intravenous infusion.
|
||||
LOP-628 [Phase 1 (Terminated)]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [41] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 43.30% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive GIST430 xenograft model. Animals were administered a single i.v. injection of the vehicle, IgG-ADC, or LOP628, LMJ729, imatinib.
|
||||
| In Vivo Model | KIT-expressing GIST430 xenograft model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [41] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 46.00% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive NCI-H1048 SCLC xenograft model. Animals were administered a single i.v. injection of the vehicle, IgG-ADC, or LOP628; twice daily 80 mg/kg oral doses of imatinib or the combination of LOP628 with imatinib.
|
||||
| In Vivo Model | KIT-expressing NCI-H1048 SCLC xenograft model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [41] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 75.00% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive GIST430 xenograft model. Animals were administered a single i.v. injection of the vehicle, IgG-ADC, or LOP628, LMJ729, imatinib.
|
||||
| In Vivo Model | KIT-expressing GIST430 xenograft model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [41] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.20% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive GIST-T1 xenograft model. Animals were administered a single i.v. injection of the vehicle, IgG-ADC, or LOP628; twice daily 80 mg/kg oral doses of imatinib or the combination of LOP628 with imatinib.
|
||||
| In Vivo Model | KIT-expressing GIST-T1 xenograft model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [41] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
82.20%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive GIST-T1 xenograft model. An efficacy study (single 0.625 mg/kg dose) in the GIST-T1 xenograft model in mice was performed.
|
||||
| In Vivo Model | KIT-expressing GIST-T1 xenograft model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [41] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.30% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive NCI-H1048 SCLC xenograft model. Animals were administered a single i.v. injection of the vehicle, IgG-ADC, or LOP628; twice daily 80 mg/kg oral doses of imatinib or the combination of LOP628 with imatinib.
|
||||
| In Vivo Model | KIT-expressing NCI-H1048 SCLC xenograft model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [41] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.30% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive NCI-H1048 SCLC xenograft model. Animals were administered a single i.v. injection of the vehicle, IgG-ADC, or LOP628; twice daily 80 mg/kg oral doses of imatinib or the combination of LOP628 with imatinib.
|
||||
| In Vivo Model | KIT-expressing NCI-H1048 SCLC xenograft model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [41] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) | < 0.50 nM | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The inhibitory activity of LOP-628 against cancer cell growth was compared with LMJ729 against cancer cell growth in vitro.
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [41] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) | < 1.00 nM | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The inhibitory activity of LOP-628 against cancer cell growth was compared with LMJ729 against cancer cell growth in vitro.
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [41] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) | > 10.00 nM | Negative KIT expression (KIT-) | ||
| Method Description |
The inhibitory activity of LOP-628 against cancer cell growth was compared with LMJ729 against cancer cell growth in vitro.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
Anti-FGFR2/4 mAb-12425 SMCC-DM1 [Investigative]
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 9.18% (Day 44) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 208.4 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 5 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | Breast cancer PDX model | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 60.63% (Day 30) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 208.4 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 10 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: CHGA-010) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 73.73% (Day 30) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 208.4 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 10 mg/kg intravenous (i.v, q3w x2) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: CHGA-010) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 81.30% (Day 44) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 208.4 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 15 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | Breast cancer PDX model | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 52.51% (Day 38) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 208.4 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 10 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | MFM223 CDX model | ||||
| In Vitro Model | Breast carcinoma | MFM-223 cells | CVCL_1408 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 63.93% (Day 22) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 171.7 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 5 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H716 CDX model | ||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H716 cells | CVCL_1581 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 66.08% (Day 22) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 171.7 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 15 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H716 CDX model | ||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H716 cells | CVCL_1581 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.41% (Day 33) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.1-0.7 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H716 cells | CVCL_1581 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.15 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.17 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Breast carcinoma | SUM52PE cells | CVCL_3425 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.20 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Breast carcinoma | MFM-223 cells | CVCL_1408 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Negative FGFR2 expression (FGFR2-) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric carcinoma | NUGC-3 cells | CVCL_1612 | ||
SNS-622-DM1 [Investigative]
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [45] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 36.75% (Day 56) | Positive ASPH expression (ASPH +++/++) | ||
| Method Description |
Mice bearing an approximately 100 mm3 tumor xenograft were intravenously injected weekly with 2.5 mg/kg of SNS-622 or SNS-622-DM1, and tumor growth was monitored.
|
||||
| In Vivo Model | Pancreatic ductal adenocarcinoma PDX model | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [45] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 55.56% (Day 22) | Positive ASPH expression (ASPH +++/++) | ||
| Method Description |
MIA PaCa2-empty vector and MIA-PaCa2-ASPH cell lines generated sc tumors in the NSG mice were treated with SNS-622-DM1 (5 mg/kg. every 7 day) and a non-relevant IgG mAb also conjugated with DM1.
|
||||
| In Vivo Model | MIA PaCa2 CDX model | ||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | MIA PaCa-2 cells | CVCL_0428 | ||
B-B4-DM1 [Investigative]
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [46] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.90% | Positive CD138 expression (CD138+++/++) | ||
| Method Description |
The in vivo antitumor activity of B-B4-DM1 was evaluated in a CD138 positive OPM1 and OPM2 MM cells xenograft models. CB-17 SCID mice were inoculated subcutaneously in the interscapular area with 5x106 OPM1 (A-B) or OPM2 (C-D) MM cells. Animals were treated daily intravenously for 3 consecutive days with either vehicle alone (PBS; n = 5), unconjugated B-B4 (13.3 ug/kg; n = 5), B-B4DM1 (150 ug DM1/kg; n = 5), or control huC242-DM1 (150 ug DM1/kg; n = 5).
Click to Show/Hide
|
||||
| In Vivo Model | Multiple myeloma PDX model (PDX: OPM2) | ||||
| In Vitro Model | Multiple myeloma | Multiple myeloma cells | Homo sapiens | ||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [46] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 27.30% | Positive CD138 expression (CD138+++/++) | ||
| Method Description |
The in vivo antitumor activity of B-B4-DM1 was evaluated in a CD138 positive OPM1 and OPM2 MM cells xenograft models. CB-17 SCID mice were inoculated subcutaneously in the interscapular area with 5x106 OPM1 (A-B) or OPM2 (C-D) MM cells. Animals were treated daily intravenously for 3 consecutive days with either vehicle alone (PBS; n = 5), unconjugated B-B4 (13.3 ug/kg; n = 5), B-B4DM1 (150 ug DM1/kg; n = 5), or control huC242-DM1 (150 ug DM1/kg; n = 5).
Click to Show/Hide
|
||||
| In Vivo Model | CD138-expressing OPM1 MM cells xenograft model | ||||
| In Vitro Model | Multiple myeloma | Multiple myeloma cells | Homo sapiens | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [46] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
25.00 nM
|
Positive CD138 expression (CD138+++/++) | ||
| Method Description |
The inhibitory activity of B-B4-DM1 against cancer cell growth was compared with B-B4 and DM1 against cancer cell growth in vitro. The cells were treated with B-B4-DM1, B-B4 and DM1 for 96 hours.
|
||||
| In Vitro Model | Plasma cell myeloma | MM1.R cells | CVCL_8794 | ||
C12G1-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 30) | High NECTIN2 expression (NECTIN2+++) | ||
| Method Description |
Treatments c12G1-DM1 at 1 mg/kg every 4th day by intraperitoneal injection.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 30) | High NECTIN2 expression (NECTIN2+++) | ||
| Method Description |
Treatments c12G1-DM1 at 5 mg/kg every 4th day by intraperitoneal injection.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 10.40% (Day 30) | High NECTIN2 expression (NECTIN2+++) | ||
| Method Description |
Treatments c12G1-DM1 at 3 mg/kg every 4th day by intraperitoneal injection.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 11.50% (Day 30) | High NECTIN2 expression (NECTIN2+++) | ||
| Method Description |
Treatments c12G1-DM1 at 1 mg/kg every 4th day by intraperitoneal injection.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian cancer | Ovarian cancer cells | Homo sapiens | ||
| Experiment 5 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 19.60% (Day 30) | High NECTIN2 expression (NECTIN2+++) | ||
| Method Description |
Treatments c12G1-DM1 at 5 mg/kg every 4th day by intraperitoneal injection.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian cancer | Ovarian cancer cells | Homo sapiens | ||
| Experiment 6 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 32.90% (Day 30) | High NECTIN2 expression (NECTIN2+++) | ||
| Method Description |
Treatments c12G1-DM1 at 1 mg/kg every 4th day by intraperitoneal injection.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian adenocarcinoma | OV-90 cells | CVCL_3768 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 46.40% (Day 30) | High NECTIN2 expression (NECTIN2+++) | ||
| Method Description |
Treatments c12G1-DM1 at 3 mg/kg every 4th day by intraperitoneal injection.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian cancer | Ovarian cancer cells | Homo sapiens | ||
| Experiment 8 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 84.40% (Day 30) | High NECTIN2 expression (NECTIN2+++) | ||
| Method Description |
Treatments c12G1-DM1 at 3 mg/kg every 4th day by intraperitoneal injection.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian adenocarcinoma | OV-90 cells | CVCL_3768 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.90% (Day 30) | High NECTIN2 expression (NECTIN2+++) | ||
| Method Description |
Treatments c12G1-DM1 at 5 mg/kg every 4th day by intraperitoneal injection.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian adenocarcinoma | OV-90 cells | CVCL_3768 | ||
NN2101-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 2.01% (Day 20) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The animal room had a controlled 12/12-h light/dark cycle (lights on at 06:00 AM), temperature (20±26 °C), and relativehumidity (50±10%). For xenograft assays, mice were anesthetized with isoflurane. Then, cells in 50% Matrigel were subcutaneously transplanted into 5-week-old female C.B-17 severe combined immunodeficiency (SCID) mice. In vivo efficacy studies were initiated when the volume of the tumors reached ~ 200 mm3. Imatinib was formulated in distilled water for oral administration. The mice were intravenously administered NN2101-DM1 three times at the indicated concentrations,2 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | GIST xenograft model | ||||
| In Vitro Model | Mast cell leukemia | HMC-1.2 cells | CVCL_H205 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 12.30% (Day 50) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The animal room had a controlled 12/12-h light/dark cycle (lights on at 06:00 AM), temperature (20±26 °C), and relativehumidity (50±10%). For xenograft assays, mice were anesthetized with isoflurane. Then, cells in 50% Matrigel were subcutaneously transplanted into 5-week-old female C.B-17 severe combined immunodeficiency (SCID) mice. In vivo efficacy studies were initiated when the volume of the tumors reached ~ 200 mm3. Imatinib was formulated in distilled water for oral administration. The mice were intravenously administered NN2101-DM1 three times at the indicated concentrations,1 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | GIST xenograft model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 20.50% (Day 27) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The animal room had a controlled 12/12-h light/dark cycle (lights on at 06:00 AM), temperature (20±26 °C), and relativehumidity (50±10%). For xenograft assays, mice were anesthetized with isoflurane. Then, cells in 50% Matrigel were subcutaneously transplanted into 5-week-old female C.B-17 severe combined immunodeficiency (SCID) mice. In vivo efficacy studies were initiated when the volume of the tumors reached ~ 200 mm3. Imatinib was formulated in distilled water for oral administration. The mice were intravenously administered NN2101-DM1 three times at the indicated concentrations,3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | SCLC xenograft model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 50.40% (Day 50) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The animal room had a controlled 12/12-h light/dark cycle (lights on at 06:00 AM), temperature (20±26 °C), and relativehumidity (50±10%). For xenograft assays, mice were anesthetized with isoflurane. Then, cells in 50% Matrigel were subcutaneously transplanted into 5-week-old female C.B-17 severe combined immunodeficiency (SCID) mice. In vivo efficacy studies were initiated when the volume of the tumors reached ~ 200 mm3. Imatinib was formulated in distilled water for oral administration. The mice were intravenously administered NN2101-DM1 three times at the indicated concentrations,1 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | GIST xenograft model | ||||
| In Vitro Model | Adult hepatocellular carcinoma | Huh-7 cells | CVCL_0336 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 61.30% (Day 60) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The animal room had a controlled 12/12-h light/dark cycle (lights on at 06:00 AM), temperature (20±26 °C), and relativehumidity (50±10%). For xenograft assays, mice were anesthetized with isoflurane. Then, cells in 50% Matrigel were subcutaneously transplanted into 5-week-old female C.B-17 severe combined immunodeficiency (SCID) mice. In vivo efficacy studies were initiated when the volume of the tumors reached ~ 200 mm3. Imatinib was formulated in distilled water for oral administration. The mice were intravenously administered NN2101-DM1 three times at the indicated concentrations,3.5 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | GIST xenograft model | ||||
| In Vitro Model | Mast cell leukemia | HMC-1.2 cells | CVCL_H205 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 61.30% (Day 60) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The animal room had a controlled 12/12-h light/dark cycle (lights on at 06:00 AM), temperature (20±26 °C), and relativehumidity (50±10%). For xenograft assays, mice were anesthetized with isoflurane. Then, cells in 50% Matrigel were subcutaneously transplanted into 5-week-old female C.B-17 severe combined immunodeficiency (SCID) mice. In vivo efficacy studies were initiated when the volume of the tumors reached ~ 200 mm3. Imatinib was formulated in distilled water for oral administration. The mice were intravenously administered NN2101-DM1 three times at the indicated concentrations,5 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | GIST xenograft model | ||||
| In Vitro Model | Mast cell leukemia | HMC-1.2 cells | CVCL_H205 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.90% (Day 50) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The animal room had a controlled 12/12-h light/dark cycle (lights on at 06:00 AM), temperature (20±26 °C), and relativehumidity (50±10%). For xenograft assays, mice were anesthetized with isoflurane. Then, cells in 50% Matrigel were subcutaneously transplanted into 5-week-old female C.B-17 severe combined immunodeficiency (SCID) mice. In vivo efficacy studies were initiated when the volume of the tumors reached ~ 200 mm3. Imatinib was formulated in distilled water for oral administration. The mice were intravenously administered NN2101-DM1 three times at the indicated concentrations,3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | GIST xenograft model | ||||
| In Vitro Model | Adult hepatocellular carcinoma | Huh-7 cells | CVCL_0336 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.10% (Day 50) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The animal room had a controlled 12/12-h light/dark cycle (lights on at 06:00 AM), temperature (20±26 °C), and relativehumidity (50±10%). For xenograft assays, mice were anesthetized with isoflurane. Then, cells in 50% Matrigel were subcutaneously transplanted into 5-week-old female C.B-17 severe combined immunodeficiency (SCID) mice. In vivo efficacy studies were initiated when the volume of the tumors reached ~ 200 mm3. Imatinib was formulated in distilled water for oral administration. The mice were intravenously administered NN2101-DM1 three times at the indicated concentrations,3 mg/kg. The mice were intraperitoneally administered Etoposide was prepared in dimethyl sulfoxide, and the solution was diluted in PBS just before administration. The mice were intraperitoneally administered etoposide for 2 cycles (3 mg/kg per day;days 15 and days 1115).
Click to Show/Hide
|
||||
| In Vivo Model | GIST xenograft model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.30% (Day 27) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The animal room had a controlled 12/12-h light/dark cycle (lights on at 06:00 AM), temperature (20±26 °C), and relativehumidity (50±10%). For xenograft assays, mice were anesthetized with isoflurane. Then, cells in 50% Matrigel were subcutaneously transplanted into 5-week-old female C.B-17 severe combined immunodeficiency (SCID) mice. In vivo efficacy studies were initiated when the volume of the tumors reached ~ 200 mm3. Imatinib was formulated in distilled water for oral administration. The mice were intravenously administered NN2101-DM1 three times at the indicated concentrations,3 mg/kg. The mice were intraperitoneally administered Etoposide was prepared in dimethyl sulfoxide, and the solution was diluted in PBS just before administration. The mice were intraperitoneally administered etoposide for 2 cycles (3 mg/kg per day;days 15 and days 1115).
Click to Show/Hide
|
||||
| In Vivo Model | SCLC xenograft model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.38 ng/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.43 ng/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-430/654 cells | CVCL_7040 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.02 ng/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
8.07 ng/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.77 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Mast cell leukemia | HMC-1.2 cells | CVCL_H205 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.18 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.52 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | Caov-3 cells | CVCL_0201 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.54 ug/mL
|
Negative KIT expression (KIT-) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.99 ug/mL
|
Negative KIT expression (KIT-) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.71 ug/mL
|
Negative KIT expression (KIT-) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Lung squamous cell carcinoma | NCI-H2170 cells | CVCL_1535 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.03 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.30 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.47 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
7.38 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Esophageal squamous cell carcinoma | TF-1 cells | CVCL_1759 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
11.84 ug/mL
|
Negative KIT expression (KIT-) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Normal | COS-7 cells | CVCL_0224 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
11.95 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Normal | HUVEC-C cells | CVCL_2959 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
12.47 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H889 cells | CVCL_1598 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
17.49 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST48 cells | CVCL_7041 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
17.63 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Lung small cell carcinoma | Ms-1 cells | CVCL_IQ55 | ||
Anti-FGFR2 mAb-10164 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 2.05% (Day 22) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 171.7 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 5 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H716 CDX model | ||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H716 cells | CVCL_1581 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 57.22% (Day 22) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 171.7 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 15 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H716 CDX model | ||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H716 cells | CVCL_1581 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 65.68% (Day 38) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 208.4 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 10 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | MFM223 CDX model | ||||
| In Vitro Model | Breast carcinoma | MFM-223 cells | CVCL_1408 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.61% (Day 33) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.14 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Breast carcinoma | SUM52PE cells | CVCL_3425 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.50 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.60 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.70 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H716 cells | CVCL_1581 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.50 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Breast carcinoma | MFM-223 cells | CVCL_1408 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Negative FGFR2 expression (FGFR2-) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric carcinoma | NUGC-3 cells | CVCL_1612 | ||
2G10 RED-432 maytansine 4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [49] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 2.75% (Day 56) | Positive PLAUR expression (PLAUR +++/++) | ||
| Method Description |
To compare the in vivo anti-tumor efficacy of the various ADC constructs, we used MDA-MB-231 xenografts in mice. Animals with tumors of approximately 100 mm3 were treated once a week for four weeks with a 10 mg/kg dose of a variety of DAR 4 ADCs or 2G10 antibody.
|
||||
| In Vivo Model | MDA-MB-231 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [49] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 500 nM | Positive PLAUR expression (PLAUR +++/++) | ||
| Method Description |
MDA-MB-231 cells (0.5x104 cells/well) were seeded in 96-well plates at the density and incubated for 120 h (5 days) with of ADCs or IgG. After 5 days of drug treatment at 37°C with 5% CO2.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
F105-SPP-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 17.58% (Day 49) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
The rear flank region of female athymic rats were implanted with 5x106 cells subcutaneously (0.2 ml of25 x106 cells/ml) on the rear flank area. When mean tumor volumes reached to 250 mm3, dosed intravenously (15 mg/kg) on days 17 and 29 after tumor cell injection.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 20.88% (Day 37) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Female athymic rats were inoculated with 5x106 HT29 cells subcutancously on the rear flank area. The animals were stratified into 13 groups, 6 animals per group based on a mean tumor volume for each group of approximately 250 mm3. On the day of grouping (day 7) each group received its initial dosing (175 ug/kg).
|
||||
| In Vivo Model | HT-29 CDX model | ||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
F105-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 17.58% (Day 49) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
The rear flank region of female athymic rats were implanted with 5x106 cells subcutaneously (0.2 ml of25 x106 cells/ml) on the rear flank area. When mean tumor volumes reached to 250 mm3, dosed intravenously (15 mg/kg) on days 17 and 29 after tumor cell injection.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
Anti-KIT NEG085?SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 20.12% (Day 28) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (1.25 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST430 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 32.28% (Day 38) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (1.25 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 39.65% (Day 28) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST430 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 69.09% (Day 28) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST430 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 72.00% (Day 41) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (0.625 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 75.90% (Day 38) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.25% (Day 38) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 79.31% (Day 21) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (1.25 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H526 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 81.81% (Day 27) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.0 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.44% (Day 21) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H526 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.51% (Day 21) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H526 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
59.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
71.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
76.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
80.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
90.00%
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
98.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.03 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.47 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.55 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.64 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.82 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
46.83 nM
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
HcHAb18-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [52] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 23.23% (Day 27) | Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
HcHAb18-DM1 (1 mg/kg, twice a week for 4 weeks) induces efficient tumor cell killing in cell line-derived models of A549 cells with CD147 expression with high expression.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [52] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 23.23% (Day 27) | Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
HcHAb18-DM1 (2 mg/kg, twice a week for 4 weeks) induces efficient tumor cell killing in cell line-derived models of A549 cells with CD147 expression with high expression.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [52] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 38.63% (Day 27) | Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
HcHAb18-DM1 (4 mg/kg, twice a week for 4 weeks) induces efficient tumor cell killing in cell line-derived models of A549 cells with CD147 expression with high expression.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [52] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 56.95% (Day 27) | Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
HcHAb18-DM1 (8 mg/kg, twice a week for 4 weeks) induces efficient tumor cell killing in cell line-derived models of A549 cells with CD147 expression with high expression.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [52] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 89.22% (Day 27) | Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
HcHAb18-DM1 (16 mg/kg, twice a week for 4 weeks) induces efficient tumor cell killing in cell line-derived models of A549 cells with CD147 expression with high expression.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [52] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.2% (Day 29) | Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
HcHAb18-DM1 (30 mg/kg, i.v. injection weekly in a total 4 doses) induces efficient tumor cell killing in cell line-derived models of NCI-H226 cells with CD147 expression with high expression.
|
||||
| In Vivo Model | NCI-H226 CDX model | ||||
| In Vitro Model | Pleural epithelioid mesothelioma | NCI-H226 cells | CVCL_1544 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [52] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.84% (Day 27) | Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
HcHAb18-DM1 (32 mg/kg, twice a week for 4 weeks) induces efficient tumor cell killing in cell line-derived models of A549 cells with CD147 expression with high expression.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [52] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.40% (Day 14) | Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
HcHAb18-DM1 (18 mg/kg, twice a week for 4 weeks) induces efficient tumor cell killing in cell line-derived models of NCI-H460 cells with CD147 expression with high expression.
|
||||
| In Vivo Model | NCI-H460 CDX model | ||||
| In Vitro Model | Lung large cell carcinoma | NCI-H460 cells | CVCL_0459 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [52] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.46 ug/mL
|
Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
In vitro efficacy of HcHAb18-DM1 versus HcHAb18 and IgG1-DM1 on six cell lines treated for 72 h.
|
||||
| In Vitro Model | Pleural epithelioid mesothelioma | NCI-H226 cells | CVCL_1544 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [52] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.77 ug/mL
|
Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
In vitro efficacy of HcHAb18-DM1 versus HcHAb18 and IgG1-DM1 on six cell lines treated for 72 h.
|
||||
| In Vitro Model | Lung large cell carcinoma | NCI-H460 cells | CVCL_0459 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [52] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.96 ug/mL
|
Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
In vitro efficacy of HcHAb18-DM1 versus HcHAb18 and IgG1-DM1 on six cell lines treated for 72 h.
|
||||
| In Vitro Model | Lung squamous cell carcinoma | NCI-H520 cells | CVCL_1566 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [52] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
18.26 ug/mL
|
Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
In vitro efficacy of HcHAb18-DM1 versus HcHAb18 and IgG1-DM1 on six cell lines treated for 72 h.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
CNTO95-SPP-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 28.74% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 3 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 44.54% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 6 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.81% (Day 37) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Female athymic rats were inoculated with 5x106 HT29 cells subcutancously on the rear flank area. The animals were stratified into 13 groups, 6 animals per group based on a mean tumor volume for each group of approximately 250 mm3. On the day of grouping (day 7) each group received its initial dosing (175 ug/kg).
|
||||
| In Vivo Model | HT-29 CDX model | ||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 4 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 71.21% (Day 24) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Nine-week-old athymic nude rats were subcutaneously inoculated with A375.S2human melanoma cells. ADC (5 mg/kg) andappropriate control compounds were intravenously injected (three injection every other day inthe first week followed by one injection per week for two weeks.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.15% (Day 24) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Nine-week-old athymic nude rats were subcutaneously inoculated with A375.S2human melanoma cells. ADC (10 mg/kg) andappropriate control compounds were intravenously injected (three injection every other day inthe first week followed by one injection per week for two weeks.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.23% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 10 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.10% (Day 49) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
The rear flank region of female athymic rats were implanted with 5x106 cells subcutaneously (0.2 ml of25 x106 cells/ml) on the rear flank area. When mean tumor volumes reached to 250 mm3, dosed intravenously (15 mg/kg) on days 17 and 29 after tumor cell injection.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.81% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 15 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.00 ug/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were seeded into white 96-well tissue culture plates (5000 cells/well)in culture medium and incubated for 16 hrs. Serial dilutions of immunoconjugates were added toeach appropraite wells (0-20 ug/ml). Tissue culture plates were incubated at 37°C for 96 hrs.
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 2 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.20 ug/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were seeded into white 96-well tissue culture plates (5000 cells/well)in culture medium and incubated for 16 hrs. Serial dilutions of immunoconjugates were added toeach appropraite wells (0-20 ug/ml). Tissue culture plates were incubated at 37°C for 96 hrs.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.19 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 4 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.27 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.27 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | A2780 cells | CVCL_0134 | ||
CNTO95-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 28.74% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 3 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 44.54% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 6 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.23% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 10 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.81% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 15 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
2G10 RED-106 maytansine 4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [49] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 30.42% (Day 56) | Positive PLAUR expression (PLAUR +++/++) | ||
| Method Description |
To compare the in vivo anti-tumor efficacy of the various ADC constructs, we used MDA-MB-231 xenografts in mice. Animals with tumors of approximately 100 mm3 were treated once a week for four weeks with a 10 mg/kg dose of a variety of DAR 4 ADCs or 2G10 antibody.
|
||||
| In Vivo Model | MDA-MB-231 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [49] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 500 nM | Positive PLAUR expression (PLAUR +++/++) | ||
| Method Description |
MDA-MB-231 cells (0.5x104 cells/well) were seeded in 96-well plates at the density and incubated for 120 h (5 days) with of ADCs or IgG. After 5 days of drug treatment at 37°C with 5% CO2.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
Anti-FGFR2/4 mAb-12422 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 30.85% (Day 22) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 171.7 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 5 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H716 CDX model | ||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H716 cells | CVCL_1581 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 35.79% (Day 22) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 171.7 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 15 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H716 CDX model | ||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H716 cells | CVCL_1581 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.52% (Day 33) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
Mirvetuximab-SPP-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
32.17% (Day 30)
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Animals with established tumors of about 130 mm3 were treated with intravenous single injection of the M9346A-DM conjugates at 2.5±0.2 mg/kg, equivalent to 51±3 ug conjugated maytansinoid per kg The conjugates were injected on day 20 after cell inoculation.
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
50.00% (Day 32)
|
High FOLR1 expression (FOLR1+++; 1,300,000 FOLR1 molecules/cell) | ||
| Method Description |
Animals with established tumors of about 130 mm3 were treated with intravenous single injection of the M9346A-DM conjugates at 2.5±0.2 mg/kg, equivalent to 51±3 ug conjugated maytansinoid per kg The conjugates were injected on day 7 after cell inoculation.
|
||||
| In Vivo Model | Ovarian carcinoma Igrov-1 CDX model | ||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.11±0.04 nM
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.13±0.08 nM
|
Moderate FOLR1 expression (FOLR1++; 290,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Gestational choriocarcinoma | JEG-3 cells | CVCL_0363 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.14±0.05 nM
|
High FOLR1 expression (FOLR1+++; 1,300,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
2G10 RED-425 maytansine 4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [49] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 38.75% (Day 56) | Positive PLAUR expression (PLAUR +++/++) | ||
| Method Description |
To compare the in vivo anti-tumor efficacy of the various ADC constructs, we used MDA-MB-231 xenografts in mice. Animals with tumors of approximately 100 mm3 were treated once a week for four weeks with a 10 mg/kg dose of a variety of DAR 4 ADCs or 2G10 antibody.
|
||||
| In Vivo Model | MDA-MB-231 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [49] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
500 nM
|
Positive PLAUR expression (PLAUR +++/++) | ||
| Method Description |
MDA-MB-231 cells (0.5x104 cells/well) were seeded in 96-well plates at the density and incubated for 120 h (5 days) with of ADCs or IgG. After 5 days of drug treatment at 37°C with 5% CO2.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
4C9-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 40.00% (Day 14) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
In vivo efficacy of 4C9-DM1 was examined using mouse models xenotransplanted with NCI-H526, mice with established tumors were randomized into different treatment groups when the tumor volume reached ~200 mm 3. The dose of 4C9-DM1 was 1 mg/kg twice at day 0 and day 7.
|
||||
| In Vivo Model | Small cell lung cancer CDX model | ||||
| In Vitro Model | Small cell lung cancer | Small cell lung cancer cells | Homo sapiens | ||
| Experiment 2 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.00% (Day 14) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
In vivo efficacy of 4C9-DM1 was examined using mouse models xenotransplanted with NCI-H526, mice with established tumors were randomized into different treatment groups when the tumor volume reached ~200 mm 3. The dose of 4C9-DM1 was 3 mg/kg twice at day 0 and day 7.
|
||||
| In Vivo Model | Small cell lung cancer CDX model | ||||
| In Vitro Model | Small cell lung cancer | Small cell lung cancer cells | Homo sapiens | ||
| Experiment 3 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 59.00% (Day 14) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
In vivo efficacy of 4C9-DM1 was examined using mouse models xenotransplanted with NCI-H526, mice with established tumors were randomized into different treatment groups when the tumor volume reached ~200 mm 3. The dose of 4C9-DM1 was 5 mg/kg twice at day 0 and day 7.
|
||||
| In Vivo Model | Small cell lung cancer CDX model | ||||
| In Vitro Model | Small cell lung cancer | Small cell lung cancer cells | Homo sapiens | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.16 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
C-Kit positive or negative SCLC cell lines were seeded into 96-well plates and incubated with 4C9 or 4C9-DM1 in a dose-dependent manner for 35 days.
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.32 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
C-Kit positive or negative SCLC cell lines were seeded into 96-well plates and incubated with 4C9 or 4C9-DM1 in a dose-dependent manner for 35 days.
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H889 cells | CVCL_1598 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.08 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
C-Kit positive or negative SCLC cell lines were seeded into 96-well plates and incubated with 4C9 or 4C9-DM1 in a dose-dependent manner for 35 days.
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
16.58 nM
|
Negative KIT expression (KIT-) | ||
| Method Description |
C-Kit positive or negative SCLC cell lines were seeded into 96-well plates and incubated with 4C9 or 4C9-DM1 in a dose-dependent manner for 35 days.
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H446 cells | CVCL_1562 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
35.50 nM
|
Negative KIT expression (KIT-) | ||
| Method Description |
C-Kit positive or negative SCLC cell lines were seeded into 96-well plates and incubated with 4C9 or 4C9-DM1 in a dose-dependent manner for 35 days.
|
||||
| In Vitro Model | Lung squamous cell carcinoma | NCI-H2170 cells | CVCL_1535 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
47.63 nM
|
Negative KIT expression (KIT-) | ||
| Method Description |
C-Kit positive or negative SCLC cell lines were seeded into 96-well plates and incubated with 4C9 or 4C9-DM1 in a dose-dependent manner for 35 days.
|
||||
| In Vitro Model | Amelanotic melanoma | MDA-MB-435 cells | CVCL_0417 | ||
M9346A-4a-Cxxx-Mal-CX-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [55] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 41.22% (Day 23) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
M9346A-4a-Cxxx-Mal-CX-DM1 (200 ug/kg, every seven days x3) induces efficient tumor cell killing in cell line-derived models of KB cells with HER2 expression with high expression.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [55] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.58% (Day 23) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
M9346A-4a-Cxxx-Mal-CX-DM1 (100 ug/kg, every seven days x3) induces efficient tumor cell killing in cell line-derived models of KB cells with HER2 expression with high expression.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [55] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.15 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Cytotoxic IC50 Values (as a Function of [ADC] and [DM1]) for Various M9346A-CX-DM1 ADCs against FR-Expressing KB Cells in the absence of 1 M unconjugated M9346A antibody.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [55] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.00 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Cytotoxic IC50 Values (as a Function of [ADC] and [DM1]) for Various M9346A-CX-DM1 ADCs against FOLR1-Expressing KB Cells in the presence of 1 M unconjugated M9346A antibody.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Anti-KIT NEG085?SPP-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 41.55% (Day 27) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.55% (Day 27) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 91.32% (Day 27) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (10 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
Anti-KIT 20376?SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 43.00% (Day 23) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (10 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 49.00% (Day 40) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (0.625 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 91.00% (Day 40) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.00% (Day 40) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.00% (Day 40) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (10 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
54.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
69.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
69.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
79.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
95.00%
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
97.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
100.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.05 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.33 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.34 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.51 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.69 nM
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
Anti-KIT NEG087?SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 44.00% (Day 41) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (0.625 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
57.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
70.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
78.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
80.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
90.00%
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
98.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.03 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.05 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.41 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.50 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.24 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
6.07 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
45.43 nM
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
Anti-FGFR2 mAb-12433 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 44.97% (Day 38) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 208.4 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 10 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | MFM223 CDX model | ||||
| In Vitro Model | Breast carcinoma | MFM-223 cells | CVCL_1408 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 91.03% (Day 33) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.69% (Day 31) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 35) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 233 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 15 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.29 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Breast carcinoma | SUM52PE cells | CVCL_3425 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
24.00 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Breast carcinoma | MFM-223 cells | CVCL_1408 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H716 cells | CVCL_1581 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Negative FGFR2 expression (FGFR2-) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric carcinoma | NUGC-3 cells | CVCL_1612 | ||
Anti-KIT NEG026?SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.00% (Day 41) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (0.625 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
TF-mAb-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 48.00% (Day 20) | Positive TF expression (TF +++) | ||
| Method Description |
Conjugates of the exemplary antibodies were tested using an established xenograft model implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 3.75 mg/kg, i.v., qw of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | HCC1806 CDX model | ||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 20) | Positive TF expression (TF +++) | ||
| Method Description |
Conjugates of the exemplary antibodies were tested using an established xenograft model implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 15 mg/kg, i.v., qw of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | HCC1806 CDX model | ||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.02 nM
|
Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | BxPC-3 cells | CVCL_0186 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07 nM
|
Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Invasive breast carcinoma | Hs 578T cells | CVCL_0332 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
17.20 nM
|
Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Glioblastoma | U-87MG cells | CVCL_0022 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
25.40 nM
|
Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
100 nM
|
Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Invasive breast carcinoma | T-47D cells | CVCL_0553 | ||
THL4-mpeoDM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [57] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 51.61% (Day 24) | Positive DLL4 expression (DLL4 +++/++) | ||
| Method Description |
Drugs were intravenously (i.v.) administered in total three times on days 1, 4 and 7. 1.5 mg/kg HLmD4 was intravenously administered once every three days for three weeks.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [57] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 57.73% (Day 24) | Positive DLL4 expression (DLL4 +++/++) | ||
| Method Description |
Drugs were intravenously (i.v.) administered in total three times on days 1, 4 and 7. 1.5 mg/kg HLmD4 was intravenously administered once every three days for three weeks.
|
||||
| In Vivo Model | MDA-MB-231 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [57] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.25% (Day 24) | Positive DLL4 expression (DLL4 +++/++) | ||
| Method Description |
Drugs were intravenously (i.v.) administered in total three times on days 1, 4 and 7. 5 mg/kg HLmD4 was intravenously administered once every three days for three weeks.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [57] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 86.11% (Day 24) | Positive DLL4 expression (DLL4 +++/++) | ||
| Method Description |
Drugs were intravenously (i.v.) administered in total three times on days 1, 4 and 7. 5 mg/kg HLmD4 was intravenously administered once every three days for three weeks.
|
||||
| In Vivo Model | MDA-MB-231 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [57] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
37.45 nM
|
Positive DLL4 expression (DLL4 +++/++) | ||
| Method Description |
In vitro cytotoxicity and mechanism of anti-DLL4 ADCs. The cytotoxicity of ADCs was assessed by MTT assay. The percentage of cell inhibition relative to untreated control HUVEC cells was calculated for each drug concentration.
|
||||
| In Vitro Model | Normal | HUVEC-C cells | CVCL_2959 | ||
Mirvetuximab-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
55.86% (Day 14)
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Animals with established tumors of about 130 mm3 were treated with intravenous single injection of the M9346A-DM conjugates at 25 02 mg/kg, equivalent to 51 3 g conjugated maytansinoid per kg The conjugates were injected on day 7 after cell inoculation.
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
99.17% (Day 10)
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Five-week-old female CB-17 severe combined immunodeficient (SCID) mice were obtained and quarantined for 7 days prior to study initiation Mice were inoculated subcutaneously with KB cells (1 x107 cells per mouse) resuspended in serum-free culture mediaMice with established KB xenografts were dosed with a single intravenous injection of 200 g of conjugated maytansinoid per kg, equivalent to 102 mg/kg antibody on day 6 after cell inoculationTumor dimensions were measured twice weekly and volume was calculated as (A B2) 05, where A represents the largest and B the perpendicular tumor diameter.
Click to Show/Hide
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.08±0.01 nM
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10±0.02 nM
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
7.00±2.00 nM
|
Moderate FOLR1 expression (FOLR1++; 290,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure) Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Gestational choriocarcinoma | JEG-3 cells | CVCL_0363 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
20.00±0.70 nM
|
High FOLR1 expression (FOLR1+++; 1,300,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
huMov19-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 56.67% (Day 20) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 2.5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 89.15% (Day 20) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.52% (Day 20) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 10 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
20.00 nM
|
Moderate FOLR1 expression (FOLR1 ++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Gestational choriocarcinoma | JEG-3 cells | CVCL_0363 | ||
Anti-KIT NEG086?SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 57.00% (Day 41) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (0.625 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.61% (Day 27) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.0 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
54.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
69.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
74.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
80.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
91.00%
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
98.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.03 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.06 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.43 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.52 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.83 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.22 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
16.92 nM
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
Anti-FGFR2/4 mAb-10918 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 61.12% (Day 33) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.20 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.61 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
Anti-FGFR2/4 mAb-11722 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 61.76% (Day 33) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.20 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.59 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
M9346A-4b-Cxxx-Mal-CX-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [55] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 62.10% (Day 23) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
M9346A-4b-Cxxx-Mal-CX-DM1 (100 ug/kg, every seven days x3) induces efficient tumor cell killing in cell line-derived models of KB cells with HER2 expression with high expression.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [55] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 73.36% (Day 23) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
M9346A-4b-Cxxx-Mal-CX-DM1 (200 ug/kg, every seven days x3) induces efficient tumor cell killing in cell line-derived models of KB cells with HER2 expression with high expression.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [55] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.20 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Cytotoxic IC50 Values (as a Function of [ADC] and [DM1]) for Various M9346A-CX-DM1 ADCs against FR-Expressing KB Cells in the absence of 1 M unconjugated M9346A antibody.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [55] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.00 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Cytotoxic IC50 Values (as a Function of [ADC] and [DM1]) for Various M9346A-CX-DM1 ADCs against FOLR1-Expressing KB Cells in the presence of 1 M unconjugated M9346A antibody.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Anti-KIT NEG027?SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 63.65% (Day 42) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (0.625 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 83.95% (Day 42) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (1.25 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 85.25% (Day 42) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
HuFR1-65-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
65.00% (Day 12)
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Five-week-old female CB-17 severe combined immunodeficient (SCID) mice were obtained and quarantined for 7 days prior to study initiation Mice were inoculated subcutaneously with KB cells (1 x107 cells per mouse) resuspended in serum-free culture mediaMice with established KB xenografts were dosed with a single intravenous injection of 200 g of conjugated maytansinoid per kg, equivalent to 102 mg/kg antibody on day 6 after cell inoculationTumor dimensions were measured twice weekly and volume was calculated as (A B2) 05, where A represents the largest and B the perpendicular tumor diameter.
Click to Show/Hide
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.08±0.02 nM
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
HuFR1-48-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
65.00% (Day 12)
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Five-week-old female CB-17 severe combined immunodeficient (SCID) mice were obtained and quarantined for 7 days prior to study initiation Mice were inoculated subcutaneously with KB cells (1 x107 cells per mouse) resuspended in serum-free culture mediaMice with established KB xenografts were dosed with a single intravenous injection of 200 g of conjugated maytansinoid per kg, equivalent to 102 mg/kg antibody on day 6 after cell inoculationTumor dimensions were measured twice weekly and volume was calculated as (A B2) 05, where A represents the largest and B the perpendicular tumor diameter.
Click to Show/Hide
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07±0.02 nM
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Anti-FGFR2 mAb-12944 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 65.73% (Day 31) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
Anti-FGFR2 mAb-10846 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 71.12% (Day 31) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.20 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.43 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
Anti-FGFR2/4 mAb-10923 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 71.79% (Day 33) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.20 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.45 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
Anti-FGFR2 mAb-11723 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 74.86% (Day 31) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.20 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.91 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
P38B-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [59] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 75.00% (Day 18) | Positive PDPN expression (PDPN +++/++) | ||
| Method Description |
One day after cell inoculation, 100 mg of antibodies (P38B-DM1, P38B, or normal canine IgG) in 100 mL of PBS were injected into the peritoneal cavity of each mouse. On days 8 and 14, 5 mg/kg P38B-DM1 was injected into each mouse.
|
||||
| In Vivo Model | CHO/dPDPN CDX model | ||||
| In Vitro Model | Melanoma | CHO cells (PDPN expression) | CVCL_0213 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [59] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 48.00 ng/mL | Positive PDPN expression (PDPN +++/++) | ||
| Method Description |
0.01-10 mg/mL for 30 minutes, followed by treatment with fluorescein isothiocyanate-conjugated.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [65] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.09 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
0.01-10 mg/mL for 30 minutes, followed by treatment with fluorescein isothiocyanate-conjugated.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [65] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.12 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
0.01-10 mg/mL for 30 minutes, followed by treatment with fluorescein isothiocyanate-conjugated.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [65] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.78 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
0.01-10 mg/mL for 30 minutes, followed by treatment with fluorescein isothiocyanate-conjugated.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [65] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
35.00 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
0.01-10 mg/mL for 30 minutes, followed by treatment with fluorescein isothiocyanate-conjugated.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [65] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
97.62 nM
|
Low HER2 expression (HER2+) | ||
| Method Description |
0.01-10 mg/mL for 30 minutes, followed by treatment with fluorescein isothiocyanate-conjugated.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [65] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
110.54 nM
|
Low HER2 expression (HER2+) | ||
| Method Description |
0.01-10 mg/mL for 30 minutes, followed by treatment with fluorescein isothiocyanate-conjugated.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
Anti-FGFR2 mAb-10220 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 76.72% (Day 33) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.20 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.33 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
Anti-KIT NEG024?SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.00% (Day 41) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (0.625 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.01% (Day 27) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.0 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.00% (Day 23) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 23) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 23) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (10 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
53.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
66.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
72.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
78.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
92.00%
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
98.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.02 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.72 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.74 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.26 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
8.49 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
22.77 nM
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
B-B4-SPP-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [60] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.45% (Day 20) | Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
MOLP-8 cells (1.5x107 cells per mouse) suspended in a 50:50 mixture of serum free media and matrigel were injected subcutaneously in the area under the right shoulder in 100 ul. Nine groups (n=6) were treated with a single intravenous injection of ADCs, each at doses of 250 ug/kg.
|
||||
| In Vivo Model | MOLP-8 CDX model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
Tmab-SPP-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.60% (Day 10) | High HER2 expression (HER2+++) | ||
| Method Description |
Mice bearing mammary tumor transplants from the MMTV-HER2 Fo5 line were given a single iv injection (10 mg/kg) of Tmab-SPP-DM1, Tmab-SSNPP-DM3, Tmab-SSNPP-DM4, Tmab-MCC-DM1, or vehicle (n=7 mice per group), and tumor growth was monitored for 25 days.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.22 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. For measurement of apoptosis, BT-474 and SK-BR-3 were exposed to trastuzumab or trastuzumab-DM for 48 h.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.26 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. For measurement of apoptosis, BT-474 and SK-BR-3 were exposed to trastuzumab or trastuzumab-DM for 48 h.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
41.37 nM
|
Negative HER2 expression (HER2-) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100.00 nM | Low HER2 expression (HER2+) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
nBT062-SPP-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [60] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.61% (Day 30) | Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
MOLP-8 cells (1.5x107 cells per mouse) suspended in a 50:50 mixture of serum free media and matrigel were injected subcutaneously in the area under the right shoulder in 100 ul. Nine groups (n=6) were treated with a single intravenous injection of ADCs, each at doses of 250 ug/kg.
|
||||
| In Vivo Model | MOLP-8 CDX model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [60] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 84.27% (Day 20) | Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
MOLP-8 cells (1.5x107 cells per mouse) suspended in a 50:50 mixture of serum free media and matrigel were injected subcutaneously in the area under the right shoulder in 100 ul. Nine groups (n=6) were treated with a single intravenous injection of ADCs, each at doses of 250 ug/kg.
|
||||
| In Vivo Model | MOLP-8 CDX model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [60] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 92.11% (Day 30) | Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
MOLP-8 cells (1.5x107 cells per mouse) suspended in a 50:50 mixture of serum free media and matrigel were injected subcutaneously in the area under the right shoulder in 100 ul. Nine groups (n=6) were treated with a single intravenous injection of ADCs, each at doses of 450 ug/kg.
|
||||
| In Vivo Model | MOLP-8 CDX model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [60] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00 nM-10.00 nM
|
Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
CD138+ MOLP-8 cells were seeded in flat bottom plates at 3000 cells/well. CD138- BJAB control cells were seeded at 1000 cells/weli. The cells were treated with nBT062-SPDB-DM4nBT062-SPP-DM1 or nBT062-SMCC-DM1 at different concentrations for five days.
|
||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [60] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Negative CD138 expression (CD138 -) | ||
| Method Description |
CD138+ MOLP-8 cells were seeded in flat bottom plates at 3000 cells/well. CD138- BJAB control cells were seeded at 1000 cells/weli. The cells were treated with nBT062-SPDB-DM4nBT062-SPP-DM1 or nBT062-SMCC-DM1 at different concentrations for five days.
|
||||
| In Vitro Model | Burkitt lymphoma | BJAB cells | CVCL_5711 | ||
Anti-FGFR2 mAb-11725 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.87% (Day 31) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.00 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.20 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
nBT062-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [60] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 78.02% (Day 30) | Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
MOLP-8 cells (1.5x107 cells per mouse) suspended in a 50:50 mixture of serum free media and matrigel were injected subcutaneously in the area under the right shoulder in 100 ul. Nine groups (n=6) were treated with a single intravenous injection of ADCs, each at doses of 250 ug/kg.
|
||||
| In Vivo Model | MOLP-8 CDX model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [60] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.32% (Day 30) | Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
MOLP-8 cells (1.5x107 cells per mouse) suspended in a 50:50 mixture of serum free media and matrigel were injected subcutaneously in the area under the right shoulder in 100 ul. Nine groups (n=6) were treated with a single intravenous injection of ADCs, each at doses of 250 ug/kg per week (five weeks).
|
||||
| In Vivo Model | MOLP-8 CDX model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [60] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.27% (Day 30) | Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
MOLP-8 cells (1.5x107 cells per mouse) suspended in a 50:50 mixture of serum free media and matrigel were injected subcutaneously in the area under the right shoulder in 100 ul. Nine groups (n=6) were treated with a single intravenous injection of ADCs, each at doses of 450 ug/kg.
|
||||
| In Vivo Model | MOLP-8 CDX model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [60] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM-1.00 nM
|
Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
CD138+ MOLP-8 cells were seeded in flat bottom plates at 3000 cells/well. CD138- BJAB control cells were seeded at 1000 cells/weli. The cells were treated with nBT062-SPDB-DM4nBT062-SPP-DM1 or nBT062-SMCC-DM1 at different concentrations for five days.
|
||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [60] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Negative CD138 expression (CD138 -) | ||
| Method Description |
CD138+ MOLP-8 cells were seeded in flat bottom plates at 3000 cells/well. CD138- BJAB control cells were seeded at 1000 cells/weli. The cells were treated with nBT062-SPDB-DM4nBT062-SPP-DM1 or nBT062-SMCC-DM1 at different concentrations for five days.
|
||||
| In Vitro Model | Burkitt lymphoma | BJAB cells | CVCL_5711 | ||
Anti-KIT 9P3?SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 78.93% (Day 14) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with Kasumi-1 cells. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (10 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | Kasumi-1 CDX model | ||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 84.35% (Day 35) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with Kasumi-1 cells. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (10 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | HMC-1 CDX model | ||||
| In Vitro Model | Mast cell leukemia | HMC-1 cells | CVCL_0003 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.00% (Day 43) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (10 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST430 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 50) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 50) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (10 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
60.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
75.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
86.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H889 cells | CVCL_1598 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
87.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1930 cells | CVCL_1507 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
91.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute megakaryoblastic leukemia | CMK-11-5 cells | CVCL_0217 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
92.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
95.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Erythroleukemia | HEL 92.1.7 cells | CVCL_2481 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
98.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | OCI-M1 cells | CVCL_2149 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Adult acute myeloid leukemia | SKNO-1 cells | CVCL_2196 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Essential thrombocythemia | UKE-1 cells | CVCL_0104 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
100.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
100.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute megakaryoblastic leukemia | M-07e cells | CVCL_2106 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.02 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.05 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute megakaryoblastic leukemia | CMK-11-5 cells | CVCL_0217 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.05 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.08 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute megakaryoblastic leukemia | M-07e cells | CVCL_2106 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.08 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 20 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.09 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1930 cells | CVCL_1507 | ||
| Experiment 21 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.11 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | OCI-M1 cells | CVCL_2149 | ||
| Experiment 22 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.15 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H889 cells | CVCL_1598 | ||
| Experiment 23 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.61 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Erythroleukemia | HEL 92.1.7 cells | CVCL_2481 | ||
| Experiment 24 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
1.29 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 25 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
1.80 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Essential thrombocythemia | UKE-1 cells | CVCL_0104 | ||
| Experiment 26 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
3.60 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Adult acute myeloid leukemia | SKNO-1 cells | CVCL_2196 | ||
| Experiment 27 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
4.00 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 28 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
4.30 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
Anti-FGFR2/4 mAb-12439 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.60% (Day 31) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
ADAPT6-ABD-mcDM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [61] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 83.30% (Day 23) | High HER2 expression (HER2+++) | ||
| Method Description |
The SKOV3 xenografts were established in female BALB/c nu/nu mice by subcutaneous implantation of 1 x107 SKOV3 cells in 100 uL of medium in the abdominal region. The therapy started one week after the implantation. The mice were randomized in three groups (n = 910). One group of mice received intravenous (i.v.) injections of 13.3 mg/kg of ADAPT6-ABD-mcDM1 in 100 uL of PBS, the second group of mice received i.v. injections of the same dose of ADAPTNeg-ABD-mcDM1 in 100 uL of PBS, and the third group of mice received i.v. injections of 100 uL of PBS.
Click to Show/Hide
|
||||
| In Vivo Model | ER2-expressing SKOV3 ovarian cancer xenograft model | ||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [70] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.00 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
The cytotoxic potential of ADAPT6-ABD-mcDM1 was investigated by incubation of dilution series of the conjugate with cell lines having different HER2-expression levels.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [70] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
7.00 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
The cytotoxic potential of ADAPT6-ABD-mcDM1 was investigated by incubation of dilution series of the conjugate with cell lines having different HER2-expression levels.
|
||||
| In Vitro Model | Breast adenocarcinoma | AU565 cells | CVCL_1074 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [70] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
80.00 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
The cytotoxic potential of ADAPT6-ABD-mcDM1 was investigated by incubation of dilution series of the conjugate with cell lines having different HER2-expression levels.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
H3L2-mpeoDM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [57] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 84.38% (Day 24) | Positive DLL4 expression (DLL4 +++/++) | ||
| Method Description |
Drugs were intravenously (i.v.) administered in total three times on days 1, 4 and 7. 5 mg/kg JmD4 was intravenously administered once every three days for three weeks.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [57] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.43% (Day 24) | Positive DLL4 expression (DLL4 +++/++) | ||
| Method Description |
Drugs were intravenously (i.v.) administered in total three times on days 1, 4 and 7. 5 mg/kg JmD4 was intravenously administered once every three days for three weeks.
|
||||
| In Vivo Model | MDA-MB-231 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [57] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
36.12 nM
|
Positive DLL4 expression (DLL4 +++/++) | ||
| Method Description |
In vitro cytotoxicity and mechanism of anti-DLL4 ADCs. The cytotoxicity of ADCs was assessed by MTT assay. The percentage of cell inhibition relative to untreated control HUVEC cells was calculated for each drug concentration.
|
||||
| In Vitro Model | Normal | HUVEC-C cells | CVCL_2959 | ||
HuFR1-49-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
85.00% (Day 12)
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Five-week-old female CB-17 severe combined immunodeficient (SCID) mice were obtained and quarantined for 7 days prior to study initiation Mice were inoculated subcutaneously with KB cells (1 x107 cells per mouse) resuspended in serum-free culture mediaMice with established KB xenografts were dosed with a single intravenous injection of 200 g of conjugated maytansinoid per kg, equivalent to 102 mg/kg antibody on day 6 after cell inoculationTumor dimensions were measured twice weekly and volume was calculated as (A B2) 05, where A represents the largest and B the perpendicular tumor diameter.
Click to Show/Hide
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07±0.01 nM
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Anti-FGFR2 mAb-12931 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 85.56% (Day 31) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
HuFR1-57-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
86.67% (Day 12)
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Five-week-old female CB-17 severe combined immunodeficient (SCID) mice were obtained and quarantined for 7 days prior to study initiation Mice were inoculated subcutaneously with KB cells (1 x107 cells per mouse) resuspended in serum-free culture mediaMice with established KB xenografts were dosed with a single intravenous injection of 200 g of conjugated maytansinoid per kg, equivalent to 102 mg/kg antibody on day 6 after cell inoculationTumor dimensions were measured twice weekly and volume was calculated as (A B2) 05, where A represents the largest and B the perpendicular tumor diameter.
Click to Show/Hide
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.15±0.02 nM
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
M9346A-CX-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [55] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.77% (Day 23) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
M9436A-CX-DM1 (200 ug/kg, every seven days x3) induces efficient tumor cell killing in cell line-derived models of KB cells with HER2 expression with high expression.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [55] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.30% (Day 23) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
M9436A-CX-DM1 (100 ug/kg, every seven days x3) induces efficient tumor cell killing in cell line-derived models of KB cells with HER2 expression with high expression.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [55] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Cytotoxic IC50 Values (as a Function of [ADC] and [DM1]) for Various M9346A-CX-DM1 ADCs against FR-Expressing KB Cells in the absence of 1 M unconjugated M9346A antibody.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [55] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
8.00 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Cytotoxic IC50 Values (as a Function of [ADC] and [DM1]) for Various M9346A-CX-DM1 ADCs against FOLR1-Expressing KB Cells in the presence of 1 M unconjugated M9346A antibody.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Anti-FGFR2 mAb-12947 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.66% (Day 31) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
huMov19-SPP-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.72% (Day 17) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 2.5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.09% (Day 17) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.20 nM
|
Moderate FOLR1 expression (FOLR1 ++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Gestational choriocarcinoma | JEG-3 cells | CVCL_0363 | ||
M9346A-SPP-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.98% (Day 20) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OVCAR-3 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
muDS6-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.50% (Day 30) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
To demonstrate the in vivo activity of the muDS6-DM1 conjugate,human tumor xenografts were established in SCID mice.A subcutaneous model of the human pancreatic cancer cell-line HPAC was developed. The dose was 27.7 mg/kg qw x2.
|
||||
| In Vivo Model | HPAC CDX model | ||||
| In Vitro Model | Pancreatic adenocarcinoma | HPAC cells | CVCL_3517 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 30) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
To demonstrate the in vivo activity of the muDS6-DM1 conjugate,human tumor xenografts were established in SCID mice.A subcutaneous model of the human cervical cancer cell-line HeLa was developed. The dose was 27.7 mg/kg qw x2.
|
||||
| In Vivo Model | HeLa CDX model | ||||
| In Vitro Model | Endocervical adenocarcinoma | HeLa cells | CVCL_0030 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 30) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
To demonstrate the in vivo activity of the muDS6-DM1 conjugate,human tumor xenografts were established in SCID mice.A subcutaneous model of the human ovarian cancer cell-line TOV-21G was developed. The dose was 27.7 mg/kg qw x2.
|
||||
| In Vivo Model | TOV-21G CDX model | ||||
| In Vitro Model | Ovarian clear cell adenocarcinoma | TOV-21G cells | CVCL_3613 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 30) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
To demonstrate the in vivo activity of the muDS6-DM1 conjugate,human tumor xenografts were established inSCID mice.A subcutaneous model of the human cervicalcarcinoma cell-line KB was developed. The dose was 150 ug/kg every day for 5 days.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 30) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
To demonstrate the in vivo activity of the muDS6-DM1 conjugate,human tumor xenografts were established inSCID mice.A subcutaneous model of the human cervicalcarcinoma cell-line KB was developed. The dose was 250 ug/kg every day for 5 days.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 30) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
To demonstrate the in vivo activity of the muDS6-DM1 conjugate,human tumor xenografts were established inSCID mice.A subcutaneous model of the human cervicalcarcinoma cell-line KB was developed. The dose was 27.7 mg/kg qw x2.
|
||||
| In Vivo Model | OVCAR-5 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-5 cells | CVCL_1628 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Ductal carcinoma | BT-483 cells | CVCL_2319 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.45 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Invasive breast carcinoma | ZR-75-1 cells | CVCL_0588 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.46 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Endocervical adenocarcinoma | WISH cells | CVCL_1909 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.67 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Endocervical adenocarcinoma | WISH cells | CVCL_1909 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.80 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | Caov-3 cells | CVCL_0201 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.40 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.61 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | Caov-3 cells | CVCL_0201 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.80 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Endocervical adenocarcinoma | HeLa cells | CVCL_0030 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.80 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Pancreatic adenocarcinoma | HPAC cells | CVCL_3517 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.84 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Pancreatic adenocarcinoma | HPAC cells | CVCL_3517 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.00 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian clear cell adenocarcinoma | TOV-21G cells | CVCL_3613 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-20 cells | CVCL_0178 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | High grade ovarian serous adenocarcinoma | Caov-4 cells | CVCL_0202 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Pancreatic adenocarcinoma | Hs 766T cells | CVCL_0334 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-5 cells | CVCL_1628 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | T-47D cells | CVCL_0553 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | ZR-75-1 cells | CVCL_0588 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.01 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 20 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
6.88 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Ovarian clear cell adenocarcinoma | TOV-21G cells | CVCL_3613 | ||
| Experiment 21 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
10.00 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
| Experiment 22 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
14.40 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-20 cells | CVCL_0178 | ||
| Experiment 23 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Ductal carcinoma | BT-483 cells | CVCL_2319 | ||
| Experiment 24 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | High grade ovarian serous adenocarcinoma | Caov-4 cells | CVCL_0202 | ||
| Experiment 25 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Endocervical adenocarcinoma | HeLa cells | CVCL_0030 | ||
| Experiment 26 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Invasive breast carcinoma | T-47D cells | CVCL_0553 | ||
| Experiment 27 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
32.00 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Pancreatic adenocarcinoma | Hs 766T cells | CVCL_0334 | ||
| Experiment 28 Reporting the Activity Date of This ADC | [62] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
846 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-5 cells | CVCL_1628 | ||
M9346A-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 20) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OVCAR-3 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 10 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
CAC10-Gly5-MAY [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [63] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 14) | High CD30 expression (CD30+++) | ||
| Method Description |
CD30 Karpas-299 cells were either transplanted subcutaneously into NSG, or into CB17.SCID mice. Mice were treated intravenously 3 times weekly with 1 mg/kg preparations, beginning one day post randomization, when the tumors had reached a size ranging between 100 and 150 mm3.
|
||||
| In Vivo Model | Non-Hodgkin's lymphoma CDX model | ||||
| In Vitro Model | ALK-positive anaplastic large cell lymphoma | Karpas-299 cells | CVCL_1324 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [63] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 16) | High CD30 expression (CD30+++) | ||
| Method Description |
CD30 Karpas-299 cells were either transplanted subcutaneously into NSG, or into CB17.SCID mice. Mice were treated intravenously 3 times weekly with 10 mg/kg ADC preparations, beginning one day post randomization, when the tumors had reached a size ranging between 100 and 150 mm3.
|
||||
| In Vivo Model | Non-Hodgkin's lymphoma CDX model | ||||
| In Vitro Model | ALK-positive anaplastic large cell lymphoma | Karpas-299 cells | CVCL_1324 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [63] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.70 ng/mL
|
High CD30 expression (CD30+++) | ||
| Method Description |
Briefly, cells were plated on 96-well plates in 75 uL growth medium and grown at 37°C in a humidified incubator in a 7.5% CO2 atmosphere. After one day incubation, 25 uL of 3.5-fold serial dilutions of each ADC in growth medium were added, typically resulting in final ADC concentrations from 20 ug/mL to 0.02 ng/mL.
|
||||
| In Vitro Model | ALK-positive anaplastic large cell lymphoma | Karpas-299 cells | CVCL_1324 | ||
Trastuzumab-AJICAP-maytansinoid [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Minimal Effective Dose (MED) | < 5.00 mg/kg | High HER2 expression (HER2+++) | ||
| Method Description |
Following the acclimatization period (1 week), the animals were stratified by body weight and randomly assigned to the following group: three trastuzumab-AJICAP-maytansinoid groups, treated with 20 mg/kg, 60 mg/kg, and 120 mg/kg. Each group consisted of five animals for blood chemistry test as well as five animals for clinical signs and body weight measurements.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer CDX model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Maximum Tolerated Dose (MTD) | > 120.00 mg/kg | High HER2 expression (HER2+++) | ||
| Method Description |
Following the acclimatization period (1 week), the animals were stratified by body weight and randomly assigned to the following group: three trastuzumab-AJICAP-maytansinoid groups, treated with 20 mg/kg, 60 mg/kg, and 120 mg/kg. Each group consisted of five animals for blood chemistry test as well as five animals for clinical signs and body weight measurements.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer CDX model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
FR1-48-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.05 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
FR1-65-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
FR1-57-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
FR1-49-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Anti-KIT 9P3?CX1-1-DM1 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
95.00 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Erythroleukemia | HEL 92.1.7 cells | CVCL_2481 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
55.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
100.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
100.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Adult acute myeloid leukemia | SKNO-1 cells | CVCL_2196 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
100.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.02 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.04 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
1.45 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
5.20 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Adult acute myeloid leukemia | SKNO-1 cells | CVCL_2196 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
7.60 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Erythroleukemia | HEL 92.1.7 cells | CVCL_2481 | ||
T-DM1-2.6 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [64] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
Positive HER2 expression (HER2+++/++) | ||
| Method Description |
Cells were cultured in a 10% fetal bovine serum (FBS)-containing RPMI 1640 medium and were planted into 96-well plates with 6000 cells per well. The plates were incubated overnight at 37°C in a 5% CO2 cell incubator.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [64] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.05 nM
|
Positive HER2 expression (HER2+++/++) | ||
| Method Description |
Cells were cultured in a 10% fetal bovine serum (FBS)-containing RPMI 1640 medium and were planted into 96-well plates with 6000 cells per well. The plates were incubated overnight at 37°C in a 5% CO2 cell incubator.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [64] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 66.00 nM | Negative HER2 expression (HER2 -) | ||
| Method Description |
Cells were cultured in a 10% fetal bovine serum (FBS)-containing RPMI 1640 medium and were planted into 96-well plates with 6000 cells per well. The plates were incubated overnight at 37°C in a 5% CO2 cell incubator.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
H32-DM1_3.8 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.05 nM±0.05 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.54 nM±0.07 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.76 nM±0.15 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
H32-DM1_3.3 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.05 nM±0.02 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.65 nM±0.04 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.65 nM±0.05 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
H32-DM1_3.7 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.06 nM±0.04 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.75 nM±0.06 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.79 nM±0.24 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
H32-DM1_3.0 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.08 nM±0.01 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.77 nM±0.15 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.84 nM±0.15 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
huMov19-PEG4-Mal-DM1 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
A PEG4-mal-DM4 conjugate in various concentrations was added to FOLR1-expressing KB cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
A PEG4-mal-DM4 conjugate in various concentrations was added to FOLR1-expressing KB cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
M9346A-4d-Cxxx-Mal-CX-DM1 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [55] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.15 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Cytotoxic IC50 Values (as a Function of [ADC] and [DM1]) for Various M9346A-CX-DM1 ADCs against FR-Expressing KB Cells in the absence of 1 M unconjugated M9346A antibody.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [55] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.00 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Cytotoxic IC50 Values (as a Function of [ADC] and [DM1]) for Various M9346A-CX-DM1 ADCs against FOLR1-Expressing KB Cells in the presence of 1 M unconjugated M9346A antibody.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
M9346A-4c-Cxxx-Mal-CX-DM1 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [55] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.15 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Cytotoxic IC50 Values (as a Function of [ADC] and [DM1]) for Various M9346A-CX-DM1 ADCs against FR-Expressing KB Cells in the absence of 1 M unconjugated M9346A antibody.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [55] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.00 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Cytotoxic IC50 Values (as a Function of [ADC] and [DM1]) for Various M9346A-CX-DM1 ADCs against FOLR1-Expressing KB Cells in the presence of 1 M unconjugated M9346A antibody.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
5E3-emtansine [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [66] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.21 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | MM1.S cells | CVCL_8792 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [66] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.42 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | NCI-H929 cells | CVCL_1600 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [66] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.30 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | INA-6 cells | CVCL_5209 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [66] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.80 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | OCI-My7 cells | CVCL_E333 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [66] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
9.90 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | OCI-My5 cells | CVCL_E332 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [66] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
12.90 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | KMS-12-BM cells | CVCL_1334 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [66] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
16.90 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Multiple myeloma | SK-MM-1 cells | CVCL_A478 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [66] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
35.30 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | OPM-2 cells | CVCL_1625 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [66] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
37.00 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | JIM3 cells | CVCL_2533 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [66] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
39.60 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | LP-1 cells | CVCL_0012 | ||
EGFRvIII-MCC-DM1 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [67] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.40 nM
|
Positive EGFR vIII expression (EGFR vIII+++/++) | ||
| Method Description |
Cells were seeded in PDL-coated 96 well plates at 375 for MMT/hEGFRvIII, 1500 for U251/hEGFRvIII, 2000 for HEK293/hEGFRvIII, or 3000 for C4-2, PC3, and T47D cells per well in complete growth media and grown overnight.
|
||||
| In Vitro Model | Normal | HEK293 cells | CVCL_0045 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [67] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.00 nM
|
Positive EGFR vIII expression (EGFR vIII+++/++) | ||
| Method Description |
Cells were seeded in PDL-coated 96 well plates at 375 for MMT/hEGFRvIII, 1500 for U251/hEGFRvIII, 2000 for HEK293/hEGFRvIII, or 3000 for C4-2, PC3, and T47D cells per well in complete growth media and grown overnight.
|
||||
| In Vitro Model | Astrocytoma | U-251MG cells | CVCL_0021 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [67] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
10.00 nM
|
Positive EGFR vIII expression (EGFR vIII+++/++) | ||
| Method Description |
Cells were seeded in PDL-coated 96 well plates at 375 for MMT/hEGFRvIII, 1500 for U251/hEGFRvIII, 2000 for HEK293/hEGFRvIII, or 3000 for C4-2, PC3, and T47D cells per well in complete growth media and grown overnight.
|
||||
| In Vitro Model | Malignant neoplasms of the mouse mammary gland | MMT-060562 cells | CVCL_4241 | ||
Cet-DM1 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.85 nM
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
HCC1954 cells (20,000) were plated in 150 mm dishes and treated with 1 nM T-DM1 (media replaced weekly). Resistant HCC1954 T-DM1R clones (HCC-TDM1R#) were obtained by single-cell cloning and continuously cultured in the presence of 1 nM T-DM1 for a total period of 5 months.
|
||||
| In Vivo Model | HCC1954/TDR CDX model (CDX: C#10) | ||||
| In Vitro Model | Breast ductal carcinoma | HCC1954 T-DM1R (T-DM1 resistance) cells | CVCL_1259 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.85 nM
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
HCC1954 cells (20,000) were plated in 150 mm dishes and treated with 1 nM T-DM1 (media replaced weekly). Resistant HCC1954 T-DM1R clones (HCC-TDM1R#) were obtained by single-cell cloning and continuously cultured in the presence of 1 nM T-DM1 for a total period of 5 months.
|
||||
| In Vivo Model | HCC1954/TDR CDX model (CDX: C#19) | ||||
| In Vitro Model | Breast ductal carcinoma | HCC1954 T-DM1R (T-DM1 resistance) cells | CVCL_1259 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.41 nM
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
HCC1954 cells (20,000) were plated in 150 mm dishes and treated with 1 nM T-DM1 (media replaced weekly). Resistant HCC1954 T-DM1R clones (HCC-TDM1R#) were obtained by single-cell cloning and continuously cultured in the presence of 1 nM T-DM1 for a total period of 5 months.
|
||||
| In Vitro Model | Breast ductal carcinoma | HCC1954 cells | CVCL_1259 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.52 nM
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
HCC1954 cells (20,000) were plated in 150 mm dishes and treated with 1 nM T-DM1 (media replaced weekly). Resistant HCC1954 T-DM1R clones (HCC-TDM1R#) were obtained by single-cell cloning and continuously cultured in the presence of 1 nM T-DM1 for a total period of 5 months.
|
||||
| In Vivo Model | HCC1954/TDR CDX model (CDX: C#20) | ||||
| In Vitro Model | Breast ductal carcinoma | HCC1954 T-DM1R (T-DM1 resistance) cells | CVCL_1259 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
7.68 nM
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
HCC1954 cells (20,000) were plated in 150 mm dishes and treated with 1 nM T-DM1 (media replaced weekly). Resistant HCC1954 T-DM1R clones (HCC-TDM1R#) were obtained by single-cell cloning and continuously cultured in the presence of 1 nM T-DM1 for a total period of 5 months.
|
||||
| In Vivo Model | HCC1954/TDR CDX model (CDX: C#29) | ||||
| In Vitro Model | Breast ductal carcinoma | HCC1954 T-DM1R (T-DM1 resistance) cells | CVCL_1259 | ||
huFR1-21-PEG4-Mal-DM1 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00 nM-10.00 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
A PEG4-mal-DM4 conjugate in various concentrations was added to FOLR1-expressing KB cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium. Antibodies and conjugates were diluted into complete RPMI medium using 3-fold dilution seriesand 100 pL were added per well.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00 nM-10.00 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
A PEG4-mal-DM4 conjugate in various concentrations was added to FOLR1-expressing KB cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium. Antibodies and conjugates were diluted into complete RPMI medium using 3-fold dilution seriesand 100 pL were added per well.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
ICAM1-DM1 ADC [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.00 nM
|
Positive ICAM1 expression (ICAM1+++/++) | ||
| Method Description |
Cells were plated in 96-well tissue culture plates (SigmaAldrich) 1 day before treatment, serum-starved, and treated with serially diluted Tras-Me-PRX (10 mg/mL antibody) for 24 h.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | BxPC-3 cells | CVCL_0186 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
9.80 nM
|
Positive ICAM1 expression (ICAM1+++/++) | ||
| Method Description |
Cells were plated in 96-well tissue culture plates (SigmaAldrich) 1 day before treatment, serum-starved, and treated with serially diluted Tras-Me-PRX (10 mg/mL antibody) for 24 h.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | PANC-1 cells | CVCL_0480 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Positive ICAM1 expression (ICAM1+++/++) | ||
| Method Description |
Cells were plated in 96-well tissue culture plates (SigmaAldrich) 1 day before treatment, serum-starved, and treated with serially diluted Tras-Me-PRX (10 mg/mL antibody) for 24 h.
|
||||
| In Vitro Model | Normal | hTERT-HPNE cells | CVCL_C466 | ||
40H3-SMCC-DM1 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
6.65 nM
|
High EGFR expression (EGFR+++) | ||
| Method Description |
1 x104 cells per well in a volume of 100 ul were plated in 96-well tissue culture plates. After 24 h, ADCs were added at the indicated concentrations. After 72 h, the medium was removed and the viability was determined using the CellTiter-Glo luminescent cell viability assay kit.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
89.60 nM
|
High EGFR expression (EGFR+++) | ||
| Method Description |
1 x104 cells per well in a volume of 100 ul were plated in 96-well tissue culture plates. After 24 h, ADCs were added at the indicated concentrations. After 72 h, the medium was removed and the viability was determined using the CellTiter-Glo luminescent cell viability assay kit.
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100.00 nM | Moderate EGFR expression (EGFR++) | ||
| Method Description |
1 x104 cells per well in a volume of 100 ul were plated in 96-well tissue culture plates. After 24 h, ADCs were added at the indicated concentrations. After 72 h, the medium was removed and the viability was determined using the CellTiter-Glo luminescent cell viability assay kit.
|
||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-20 cells | CVCL_0178 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100.00 nM | Negative EGFR expression (EGFR-) | ||
| Method Description |
1 x104 cells per well in a volume of 100 ul were plated in 96-well tissue culture plates. After 24 h, ADCs were added at the indicated concentrations. After 72 h, the medium was removed and the viability was determined using the CellTiter-Glo luminescent cell viability assay kit.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
ch735-Py-DM1 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [72] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
50.00 nM
|
Positive polySia expression (polySia +++/++) | ||
| Method Description |
Cells were plated at 5,000, 2,500, and 2,500 cells/well, respectively, and allowed to rest for 24 h. Five-fold serial dilutions of the antibodies were added starting at 150 nM and incubated for 72-144 h.
|
||||
| In Vitro Model | Bone marrow neuroblastoma | SH-SY5Y cells | CVCL_0019 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [72] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Negative polySia expression (polySia -) | ||
| Method Description |
Cells were plated at 5,000, 2,500, and 2,500 cells/well, respectively, and allowed to rest for 24 h. Five-fold serial dilutions of the antibodies were added starting at 150 nM and incubated for 72-144 h.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
T-Py-DM1 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [72] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Negative HER2 expression (HER2 -) | ||
| Method Description |
Cells were plated at 5,000, 2,500, and 2,500 cells/well, respectively, and allowed to rest for 24 h. Five-fold serial dilutions of the antibodies were added starting at 150 nM and incubated for 72-144 h.
|
||||
| In Vitro Model | Bone marrow neuroblastoma | SH-SY5Y cells | CVCL_0019 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [72] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
144 nM
|
Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
Cells were plated at 5,000, 2,500, and 2,500 cells/well, respectively, and allowed to rest for 24 h. Five-fold serial dilutions of the antibodies were added starting at 150 nM and incubated for 72-144 h.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
2G10 RED-106 maytansine 2 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [49] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 500 nM | Positive PLAUR expression (PLAUR +++/++) | ||
| Method Description |
MDA-MB-231 cells (0.5x104 cells/well) were seeded in 96-well plates at the density and incubated for 120 h (5 days) with of ADCs or IgG. After 5 days of drug treatment at 37°C with 5% CO2.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
Tras-Gly5-May [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [63] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
18.00 ng/mL
|
High HER2 expression (HER2+++; 694,000 HER2 molecules/cell) | ||
| Method Description |
Briefly, cells were plated on 96-well plates in 75 uL growth medium and grown at 37°C in a humidified incubator in a 7.5% CO2 atmosphere. After one day incubation, 25 uL of 3.5-fold serial dilutions of each ADC in growth medium were added, typically resulting in final ADC concentrations from 20 ug/mL to 0.02 ng/mL.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [63] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 110.00 ng/mL | Moderate HER2 expression (HER2++; 32,000 HER2 molecules/cell) | ||
| Method Description |
Briefly, cells were plated on 96-well plates in 75 uL growth medium and grown at 37°C in a humidified incubator in a 7.5% CO2 atmosphere. After one day incubation, 25 uL of 3.5-fold serial dilutions of each ADC in growth medium were added, typically resulting in final ADC concentrations from 20 ug/mL to 0.02 ng/mL.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
T-FcBP-DM1 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [73] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
81.64 ng/mL
|
Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [73] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 800 ng/mL | Negative HER2 expression (HER2 -) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
GABRP Ab-DM1 ADC [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [74] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
100 ng/mL - 500 ng/mL
|
Positive GABRP expression (GABRP+++/++) | ||
| Method Description |
Cells were plated at 2000 cells/well in triplicate in 96-well plates and exposed to GABRP Ab-DM1 ADC and control IgG-DM1 for 3 days.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [74] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
100 ng/mL - 500 ng/mL
|
Positive GABRP expression (GABRP+++/++) | ||
| Method Description |
Cells were plated at 2000 cells/well in triplicate in 96-well plates and exposed to GABRP Ab-DM1 ADC and control IgG-DM1 for 3 days.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [74] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 500 ng/mL | Positive GABRP expression (GABRP+++/++) | ||
| Method Description |
Cells were plated at 2000 cells/well in triplicate in 96-well plates and exposed to GABRP Ab-DM1 ADC and control IgG-DM1 for 3 days.
|
||||
| In Vitro Model | Breast ductal carcinoma | HCC1143 cells | CVCL_1245 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [74] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 500 ng/mL | Positive GABRP expression (GABRP+++/++) | ||
| Method Description |
Cells were plated at 2000 cells/well in triplicate in 96-well plates and exposed to GABRP Ab-DM1 ADC and control IgG-DM1 for 3 days.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [74] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 500 ng/mL | Positive GABRP expression (GABRP+++/++) | ||
| Method Description |
Cells were plated at 2000 cells/well in triplicate in 96-well plates and exposed to GABRP Ab-DM1 ADC and control IgG-DM1 for 3 days.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
References
