Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0ATULB
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Laprituximab emtansine
|
|||||
| Synonyms |
IMGN 289; IMGN-289; IMGN2 89; IMGN289; J2898A-SMCC-DM1; Laprituximab emtansine
Click to Show/Hide
|
|||||
| Organization |
ImmunoGen, Inc.
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3 to 4
|
|||||
| Structure |
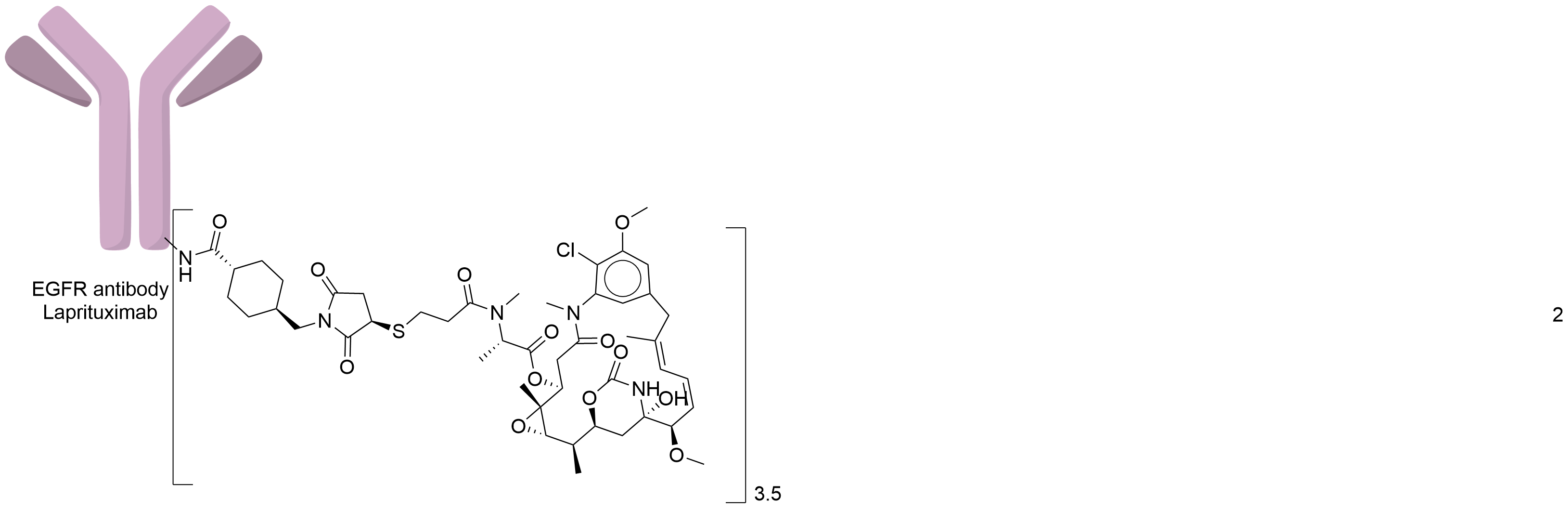
|
|||||
| Antibody Name |
Laprituximab
|
Antibody Info | ||||
| Antigen Name |
Epidermal growth factor receptor (EGFR)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM1
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC)
|
Linker Info | ||||
| Conjugate Type |
Random Lysines
|
|||||
| Combination Type |
emtansine
|
|||||
| Puchem SID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Cell Line-derived Xenograft Model
Revealed Based on the Cell Line Data
| Standard Type | Value | Units | Cell Line | Disease Model |
|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) |
0.25
|
nM
|
HNSCC cells
|
Head and neck squamous cell carcinoma
|
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT01963715 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, multi-center, open-label study of IMGN289 administered intravenously in adult patients with EGFR-positive solid tumors. | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 58.30% | High EGFR expression (EGFR+++/++) | ||
| Method Description |
The cytotoxic activity of IMGN289 was evaluated against a panel of SCCHN cell lines in vitro. Immunodeficient mice bearing established subcutaneous xenograft tumors were treated with a single intravenous injection of IMGN289 at 1,2.5 or 5.0 mg/kg (based on antibody concentration). IMGN289 dose was 5 mg/kg administered as a single injection.
|
||||
| In Vivo Model | Head and neck squamous cell carcinomas CDX model | ||||
| In Vitro Model | Hypopharyngeal squamous cell carcinoma | FaDu cells | CVCL_1218 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 83.30% | High EGFR expression (EGFR+++/++) | ||
| Method Description |
The cytotoxic activity of IMGN289 was evaluated against a panel of SCCHN cell lines in vitro. Immunodeficient mice bearing established subcutaneous xenograft tumors were treated with a single intravenous injection of IMGN289 at 1,2.5 or 5.0 mg/kg (based on antibody concentration). IMGN289 dose was 5 mg/kg administered as a single injection.
|
||||
| In Vivo Model | Head and neck squamous cell carcinomas CDX model | ||||
| In Vitro Model | Oral cavity squamous cell carcinoma | HSC-2 cells | CVCL_1287 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Minimal Effective Dose (MED) | 1.00 mg/kg | High EGFR expression (EGFR+++/++) | ||
| Method Description |
The cytotoxic activity of IMGN289 was evaluated against a panel of SCCHN cell lines in vitro. Immunodeficient mice bearing established subcutaneous xenograft tumors were treated with a single intravenous injection of IMGN289 at 1,2.5 or 5.0 mg/kg (based on antibody concentration).
|
||||
| In Vivo Model | Head and neck squamous cell carcinomas CDX model | ||||
| In Vitro Model | Hypopharyngeal squamous cell carcinoma | FaDu cells | CVCL_1218 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Minimal Effective Dose (MED) | 2.50 mg/kg | High EGFR expression (EGFR+++/++) | ||
| Method Description |
The cytotoxic activity of IMGN289 was evaluated against a panel of SCCHN cell lines in vitro. Immunodeficient mice bearing established subcutaneous xenograft tumors were treated with a single intravenous injection of IMGN289 at 1,2.5 or 5.0 mg/kg (based on antibody concentration).
|
||||
| In Vivo Model | Head and neck squamous cell carcinomas CDX model | ||||
| In Vitro Model | Oral cavity squamous cell carcinoma | HSC-2 cells | CVCL_1287 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.25 nM | High EGFR expression (EGFR+++/++) | ||
| Method Description |
Effects of IMGN289 on clonogenicity and proliferation were tested in HNSCC cell lines with varying cetuximab and gefitinib sensitivities.
|
||||
| In Vitro Model | Head and neck squamous cell carcinoma | HNSCC cells | Homo sapiens | ||
References
