Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0XRAON
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
LOP-628
|
|||||
| Synonyms |
Anti-c-kit humanised IgG1 kappa antibody-maitansine conjugate (Novartis Pharma AG); Anti-c-kit humanised IgG1 kappa antibody-maytansine conjugate; Anti-c-kit humanised IgG1kappa antibody-maitansine conjugate; Anti-proto-oncogene protein; Anti-proto-oncogene protein c-kit IgG1 kappa antibody-maitansine conjugate; LOP 628; LOP-628; LOP62 8; LOP628
Click to Show/Hide
|
|||||
| Organization |
Novartis Pharma AG; Novartis AG
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3.5
|
|||||
| Structure |
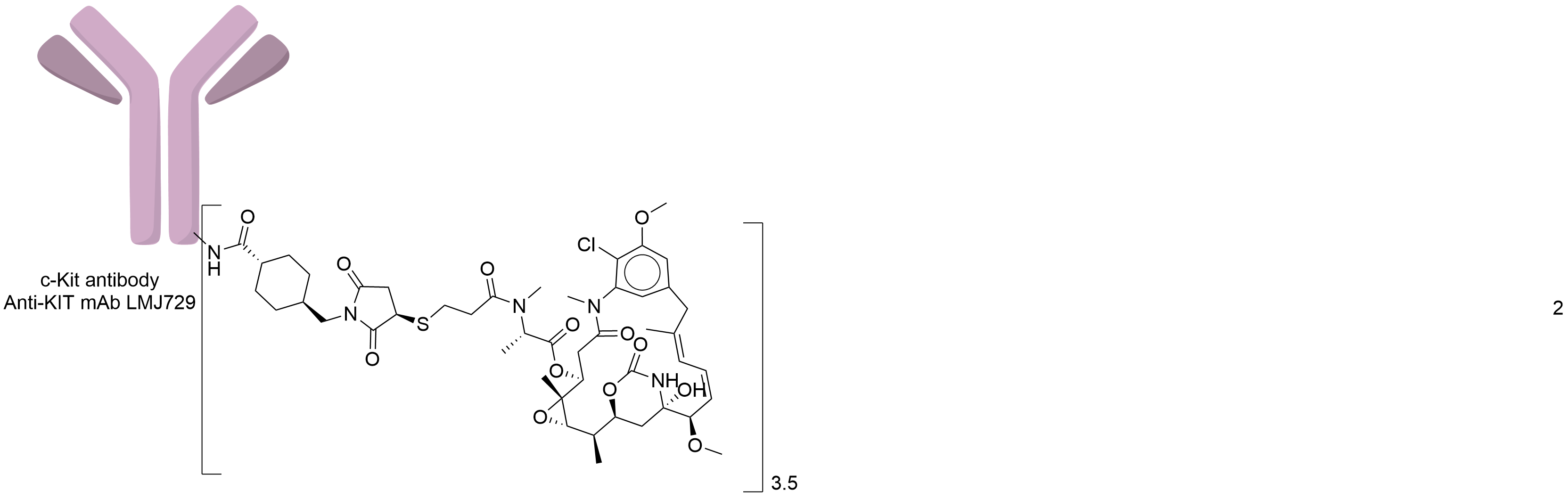
|
|||||
| Antibody Name |
humanized Anti-KIT mAb LMJ729
|
Antibody Info | ||||
| Antigen Name |
Mast/stem cell growth factor receptor Kit (KIT)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM1
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC)
|
Linker Info | ||||
| Conjugate Type |
Random Lysines
|
|||||
| Combination Type |
emtansine
|
|||||
General Information of The Activity Data Related to This ADC
Discovered Using Cell Line-derived Xenograft Model
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 43.30% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive GIST430 xenograft model. Animals were administered a single i.v. injection of the vehicle, IgG-ADC, or LOP628, LMJ729, imatinib.
|
||||
| In Vivo Model | KIT-expressing GIST430 xenograft model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 46.00% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive NCI-H1048 SCLC xenograft model. Animals were administered a single i.v. injection of the vehicle, IgG-ADC, or LOP628; twice daily 80 mg/kg oral doses of imatinib or the combination of LOP628 with imatinib.
|
||||
| In Vivo Model | KIT-expressing NCI-H1048 SCLC xenograft model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 75.00% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive GIST430 xenograft model. Animals were administered a single i.v. injection of the vehicle, IgG-ADC, or LOP628, LMJ729, imatinib.
|
||||
| In Vivo Model | KIT-expressing GIST430 xenograft model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.20% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive GIST-T1 xenograft model. Animals were administered a single i.v. injection of the vehicle, IgG-ADC, or LOP628; twice daily 80 mg/kg oral doses of imatinib or the combination of LOP628 with imatinib.
|
||||
| In Vivo Model | KIT-expressing GIST-T1 xenograft model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 82.20% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive GIST-T1 xenograft model. An efficacy study (single 0.625 mg/kg dose) in the GIST-T1 xenograft model in mice was performed.
|
||||
| In Vivo Model | KIT-expressing GIST-T1 xenograft model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.30% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive NCI-H1048 SCLC xenograft model. Animals were administered a single i.v. injection of the vehicle, IgG-ADC, or LOP628; twice daily 80 mg/kg oral doses of imatinib or the combination of LOP628 with imatinib.
|
||||
| In Vivo Model | KIT-expressing NCI-H1048 SCLC xenograft model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.30% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive NCI-H1048 SCLC xenograft model. Animals were administered a single i.v. injection of the vehicle, IgG-ADC, or LOP628; twice daily 80 mg/kg oral doses of imatinib or the combination of LOP628 with imatinib.
|
||||
| In Vivo Model | KIT-expressing NCI-H1048 SCLC xenograft model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) | < 0.50 nM | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The inhibitory activity of LOP-628 against cancer cell growth was compared with LMJ729 against cancer cell growth in vitro.
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) | < 1.00 nM | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The inhibitory activity of LOP-628 against cancer cell growth was compared with LMJ729 against cancer cell growth in vitro.
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) | > 10.00 nM | Negative KIT expression (KIT-) | ||
| Method Description |
The inhibitory activity of LOP-628 against cancer cell growth was compared with LMJ729 against cancer cell growth in vitro.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
References
