Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0VMKDY
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
muDS6-DM1
|
|||||
| Synonyms |
muDS6DM1
Click to Show/Hide
|
|||||
| Organization |
Sanofi SA
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 5 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
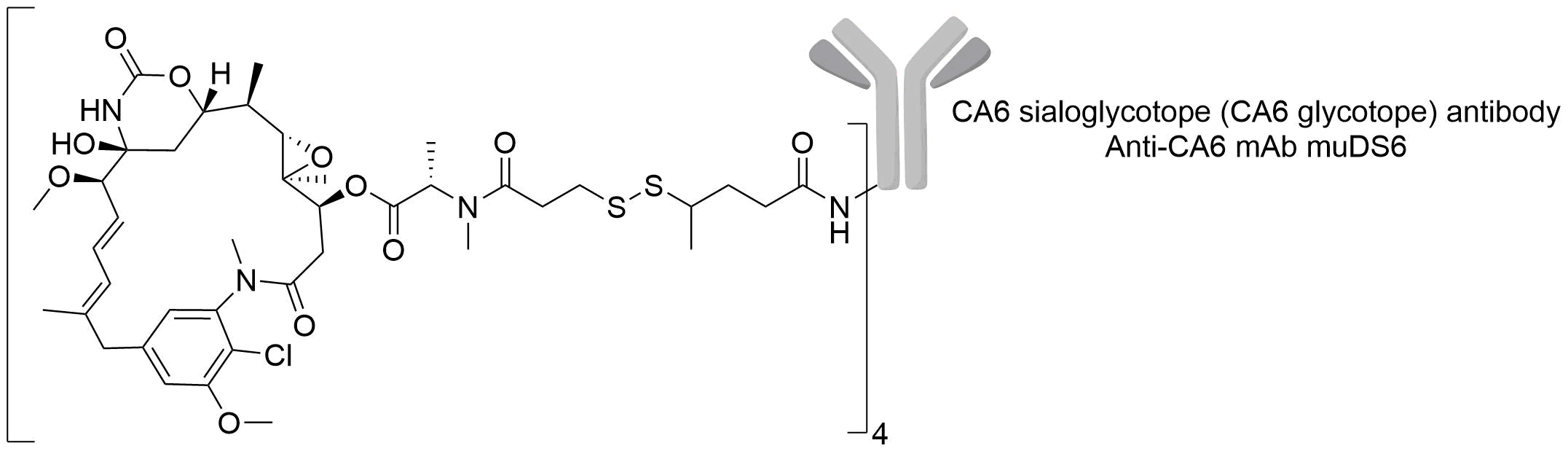
|
|||||
| Antibody Name |
Anti-CA6 mAb muDS6
|
Antibody Info | ||||
| Antigen Name |
Mucin-1 (MUC1)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM1
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
N-succinimidyl 4-(2-pyridyldithio) pentanoate (SPP)
|
Linker Info | ||||
| Conjugate Type |
Random conjugation through nucleophilic lysines.
|
|||||
| Combination Type |
Mertansine
|
|||||
General Information of The Activity Data Related to This ADC
Discovered Using Cell Line-derived Xenograft Model
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.50% (Day 30) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
To demonstrate the in vivo activity of the muDS6-DM1 conjugate,human tumor xenografts were established in SCID mice.A subcutaneous model of the human pancreatic cancer cell-line HPAC was developed. The dose was 27.7 mg/kg qw x2.
|
||||
| In Vivo Model | HPAC CDX model | ||||
| In Vitro Model | Pancreatic adenocarcinoma | HPAC cells | CVCL_3517 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 30) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
To demonstrate the in vivo activity of the muDS6-DM1 conjugate,human tumor xenografts were established in SCID mice.A subcutaneous model of the human cervical cancer cell-line HeLa was developed. The dose was 27.7 mg/kg qw x2.
|
||||
| In Vivo Model | HeLa CDX model | ||||
| In Vitro Model | Endocervical adenocarcinoma | HeLa cells | CVCL_0030 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 30) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
To demonstrate the in vivo activity of the muDS6-DM1 conjugate,human tumor xenografts were established in SCID mice.A subcutaneous model of the human ovarian cancer cell-line TOV-21G was developed. The dose was 27.7 mg/kg qw x2.
|
||||
| In Vivo Model | TOV-21G CDX model | ||||
| In Vitro Model | Ovarian clear cell adenocarcinoma | TOV-21G cells | CVCL_3613 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 30) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
To demonstrate the in vivo activity of the muDS6-DM1 conjugate,human tumor xenografts were established inSCID mice.A subcutaneous model of the human cervicalcarcinoma cell-line KB was developed. The dose was 150 ug/kg every day for 5 days.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 30) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
To demonstrate the in vivo activity of the muDS6-DM1 conjugate,human tumor xenografts were established inSCID mice.A subcutaneous model of the human cervicalcarcinoma cell-line KB was developed. The dose was 250 ug/kg every day for 5 days.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 30) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
To demonstrate the in vivo activity of the muDS6-DM1 conjugate,human tumor xenografts were established inSCID mice.A subcutaneous model of the human cervicalcarcinoma cell-line KB was developed. The dose was 27.7 mg/kg qw x2.
|
||||
| In Vivo Model | OVCAR-5 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-5 cells | CVCL_1628 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.10 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Ductal carcinoma | BT-483 cells | CVCL_2319 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.45 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Invasive breast carcinoma | ZR-75-1 cells | CVCL_0588 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.46 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Endocervical adenocarcinoma | WISH cells | CVCL_1909 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.67 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Endocervical adenocarcinoma | WISH cells | CVCL_1909 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.80 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | Caov-3 cells | CVCL_0201 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 1.40 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 1.61 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | Caov-3 cells | CVCL_0201 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 1.80 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Endocervical adenocarcinoma | HeLa cells | CVCL_0030 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 1.80 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Pancreatic adenocarcinoma | HPAC cells | CVCL_3517 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 1.84 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Pancreatic adenocarcinoma | HPAC cells | CVCL_3517 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 2.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian clear cell adenocarcinoma | TOV-21G cells | CVCL_3613 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-20 cells | CVCL_0178 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | High grade ovarian serous adenocarcinoma | Caov-4 cells | CVCL_0202 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Pancreatic adenocarcinoma | Hs 766T cells | CVCL_0334 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-5 cells | CVCL_1628 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | T-47D cells | CVCL_0553 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | ZR-75-1 cells | CVCL_0588 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 3.01 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 20 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 6.88 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Ovarian clear cell adenocarcinoma | TOV-21G cells | CVCL_3613 | ||
| Experiment 21 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 10.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
| Experiment 22 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 14.40 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-20 cells | CVCL_0178 | ||
| Experiment 23 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Ductal carcinoma | BT-483 cells | CVCL_2319 | ||
| Experiment 24 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | High grade ovarian serous adenocarcinoma | Caov-4 cells | CVCL_0202 | ||
| Experiment 25 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Endocervical adenocarcinoma | HeLa cells | CVCL_0030 | ||
| Experiment 26 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Invasive breast carcinoma | T-47D cells | CVCL_0553 | ||
| Experiment 27 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 32.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Pancreatic adenocarcinoma | Hs 766T cells | CVCL_0334 | ||
| Experiment 28 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 846 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-5 cells | CVCL_1628 | ||
References
