Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0LQZSE
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Naratuximab emtansine
|
|||||
| Synonyms |
DEBIO 1562; Debio 1562, K7153 A, IMGN5 29; IMGN 529; IMGN-529; IMGN529; K7153A-SMCC-DM1; Naratuximab emtansine
Click to Show/Hide
|
|||||
| Organization |
ImmunoGen, Inc.; Debiopharm International SA
|
|||||
| Drug Status |
Phase 2 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3 to 4
|
|||||
| Structure |
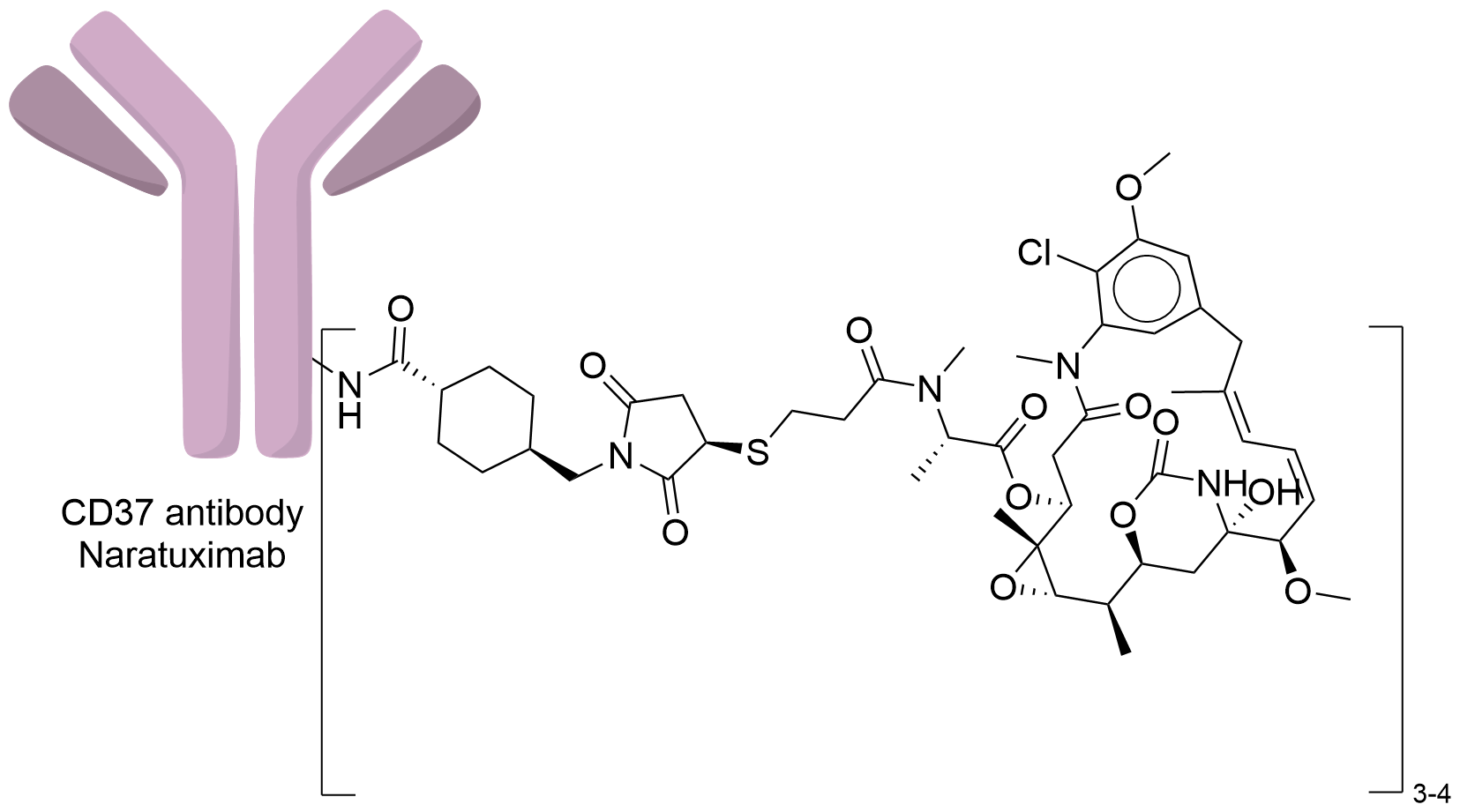
|
|||||
| Antibody Name |
Naratuximab
|
Antibody Info | ||||
| Antigen Name |
Leukocyte antigen CD37 (CD37)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM1
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC)
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
emtansine
|
|||||
| Special Approval(s) |
Orphan drug(FDA)
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| DrugMap ID | ||||||
| TTD ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Cell Line-derived Xenograft Model
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 12.82% | Positive CD38 expression (CD38+++/++) | ||
| Patients Enrolled |
Lymphoma limited to diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), or marginal zone lymphoma (MZL). Patients were also required to have received at least one prior anti-CD20 based therapeutic regimen, have a life expectancy of greater than 3 months, an Eastern Cooperative Oncology Group Performance status of 2 or lower, and adequate hematological, renal, and hepatic function.
Click to Show/Hide
|
||||
| Administration Dosage |
Conventional 3+3 dose-escalation design; intravenously once every 3 weeks; from 0.10 to 1.80 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01534715 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, multi-center, open-label study of IMGN529 administered intravenously in adult patients with relapsed or refractory non-Hodgkin lymphoma and chronic lymphocytic leukemia. | ||||
| Primary Endpoint |
A total of five objective responses were observed, resulting in an overall response rate (ORR) of 12.82% (N=39). Four of these (1 complete response [CR] and 3 partial responses [PRs]) occurred in patients with DLBCL, for an ORR of 22% in this lymphoma subset.
|
||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 14.50% (Day 38) | Positive CD38 expression (CD38+++/++) | ||
| Method Description |
IMGN529 induces efficient tumor cell killing in cell line-derived models B-cell NHL of cells with lower CD27 expression, dosed at 2.5 ug/kg , 2 qw3.
|
||||
| In Vivo Model | CD37-positive NHL and CLL model | ||||
| In Vitro Model | Non-Hodgkin lymphoma | Non-Hodgkin lymphoma cells | Homo sapiens | ||
| Chronic lymphocytic leukemia | Chronic lymphocytic leukemia cells | Homo sapiens | |||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.00% (Day 38) | Positive CD38 expression (CD38+++/++) | ||
| Method Description |
IMGN529 induces efficient tumor cell killing in cell line-derived models B-cell NHL of cells with lower CD27 expression, dosed at 5 ug/kg , 2 qw3.
|
||||
| In Vivo Model | CD37-positive NHL and CLL model | ||||
| In Vitro Model | Non-Hodgkin lymphoma | Non-Hodgkin lymphoma cells | Homo sapiens | ||
| Chronic lymphocytic leukemia | Chronic lymphocytic leukemia cells | Homo sapiens | |||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 38) | Positive CD38 expression (CD38+++/++) | ||
| Method Description |
IMGN529 induces efficient tumor cell killing in cell line-derived models B-cell NHL of cells with lower CD27 expression, dosed at 10 ug/kg , 2 qw3.
|
||||
| In Vivo Model | CD37-positive NHL and CLL model | ||||
| In Vitro Model | Non-Hodgkin lymphoma | Non-Hodgkin lymphoma cells | Homo sapiens | ||
| Chronic lymphocytic leukemia | Chronic lymphocytic leukemia cells | Homo sapiens | |||
| Experiment 4 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 100.00% (10 mg/kg) | Positive CD38 expression (CD38+++/++) | ||
| Method Description |
The inhibitory activity of SGN-LIV1A against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 1 day.
|
||||
| In Vivo Model | DoHH2 CDX model | ||||
| In Vitro Model | Diffuse large B-cell lymphoma | WSU-NHL cells | CVCL_1793 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.10 nM | |||
| Method Description |
In vitro cytotoxicity was measured by incubating 5000 target cells with indicated agents in complete RPMI-1640 media for 5 days at 37°C. The viability of remaining cells was determined by a colorimetric WST-10 assay.
|
||||
| In Vivo Model | DoHH2 CDX model | ||||
| In Vitro Model | Diffuse large B-cell lymphoma germinal center B-cell type | DoHH2 cells | CVCL_1179 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.10 nM | |||
| Method Description |
In vitro cytotoxicity was measured by incubating 5000 target cells with indicated agents in complete RPMI-1640 media for 5 days at 37°C. The viability of remaining cells was determined by a colorimetric WST-11 assay.
|
||||
| In Vivo Model | DoHH2 CDX model | ||||
| In Vitro Model | Mantle cell lymphoma | Granta-519 cells | CVCL_1818 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.10 nM | |||
| Method Description |
In vitro cytotoxicity was measured by incubating 5000 target cells with indicated agents in complete RPMI-1640 media for 5 days at 37°C. The viability of remaining cells was determined by a colorimetric WST-8 assay.
|
||||
| In Vivo Model | DoHH2 CDX model | ||||
| In Vitro Model | Burkitt lymphoma | BJAB cells | CVCL_5711 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.10 nM | Positive CD38 expression (CD38+++/++) | ||
| Method Description |
In vitro cytotoxicity was measured by incubating 5000 target cells with indicated agents in complete RPMI-1640 media for 5 days at 37°C. The viability of remaining cells was determined by a colorimetric WST-9 assay.
|
||||
| In Vivo Model | DoHH2 CDX model | ||||
| In Vitro Model | Diffuse large B-cell lymphoma | Farage cells | CVCL_3302 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 1.00 nM | |||
| Method Description |
In vitro cytotoxicity was measured by incubating 5000 target cells with indicated agents in complete RPMI-1640 media for 5 days at 37°C. The viability of remaining cells was determined by a colorimetric WST-12 assay.
|
||||
| In Vivo Model | DoHH2 CDX model | ||||
| In Vitro Model | Mantle cell lymphoma | JVM-2 cells | CVCL_1319 | ||
References
