Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0OJHEN
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
AVID-100
|
|||||
| Synonyms |
AVID 100; AVID-100; AVID100
Click to Show/Hide
|
|||||
| Organization |
Bristol Myers Squibb Co.; Formation Biologics
|
|||||
| Drug Status |
Phase 1/2 (Terminated)
|
|||||
| Indication |
In total 4 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
Undisclosed
|
|||||
| Structure |
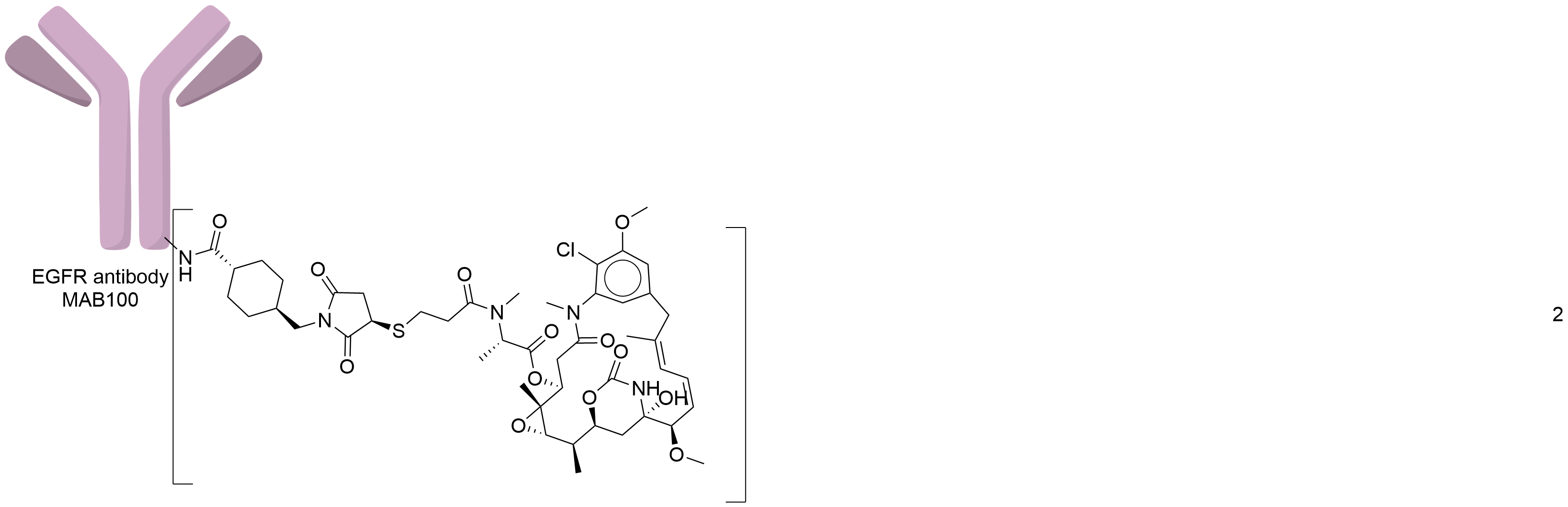
|
|||||
| Antibody Name |
MAB100
|
Antibody Info | ||||
| Antigen Name |
Epidermal growth factor receptor (EGFR)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM1
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC)
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Combination Type |
emtansine
|
|||||
| Puchem SID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Patients Enrolled |
Patients with advanced or metastatic epithelial malignancies.
|
||||
| Administration Dosage |
20, 40, 80, 120, 180, and 220 mg/m2 every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03094169 | Clinical Status | Phase 1/2 | ||
| Clinical Description | Phase 1a/2a dose escalation trial to determine safety, tolerance, MTD, and preliminary antineoplastic activity of AVID100, in patients with advanced or metastatic solid tumors of epithelial origin. | ||||
References
