Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0EPORB
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
B-003
|
|||||
| Synonyms |
Anti-HER2 monoclonal antibody-MCC-DM1 conjugate; B 003; B-003; B003
Click to Show/Hide
|
|||||
| Organization |
Shanghai Jiaolian Drug Development Co. Ltd.; Shanghai Pharmaceuticals Holding Co., Ltd.
|
|||||
| Drug Status |
Phase 1
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
Undisclosed
|
|||||
| Structure |
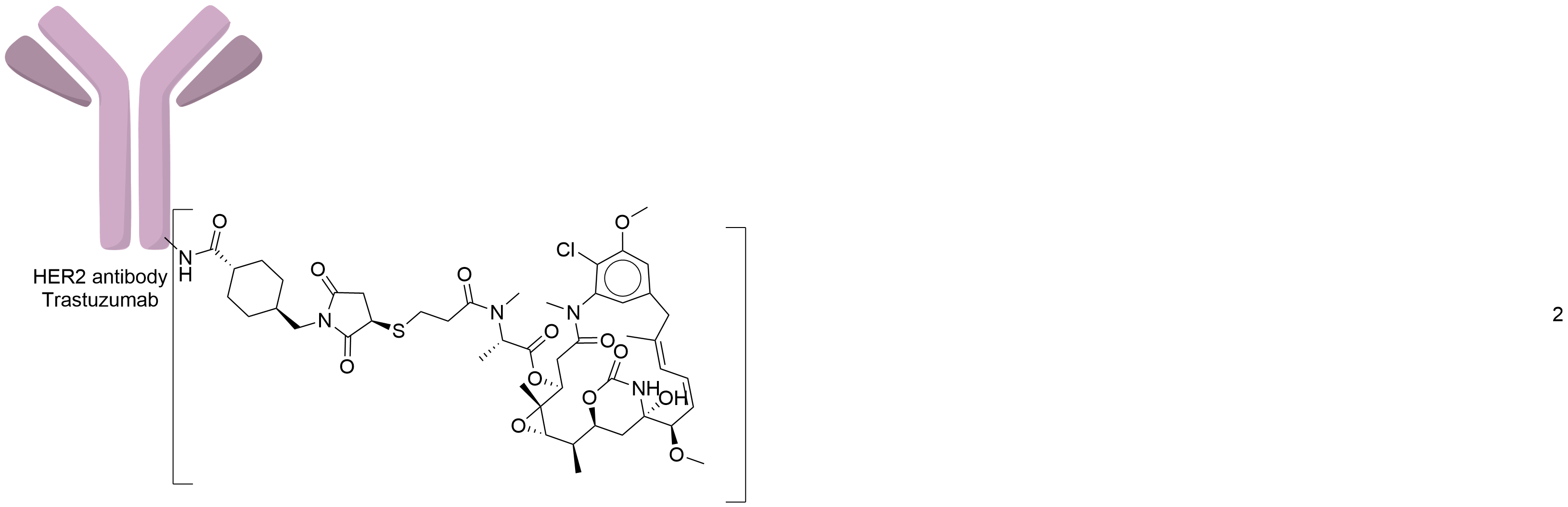
|
|||||
| Antibody Name |
Trastuzumab
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM1
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC)
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Combination Type |
emtansine
|
|||||
General Information of The Activity Data Related to This ADC
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03953833 | Clinical Status | Phase 1 | ||
| Clinical Description | Phase 1 clinical trial on the safety, tolerability, pharmacokinetics of B003 in the treatment of HER2-positive recurrent or metastatic breast cancer. | ||||
References
