Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0BBYHA
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Bivatuzumab mertansine
|
|||||
| Synonyms |
Anti-CD44v6-DM1 immunoconjugate; BIW1-1; BIWI-1; Bivatuzumab mertansine; Monoclonal antibody CD44v6-DM1 immunoconjugate
Click to Show/Hide
|
|||||
| Organization |
ImmunoGen, Inc.; Boehringer Ingelheim GmbH
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 3 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3 to 4
|
|||||
| Structure |
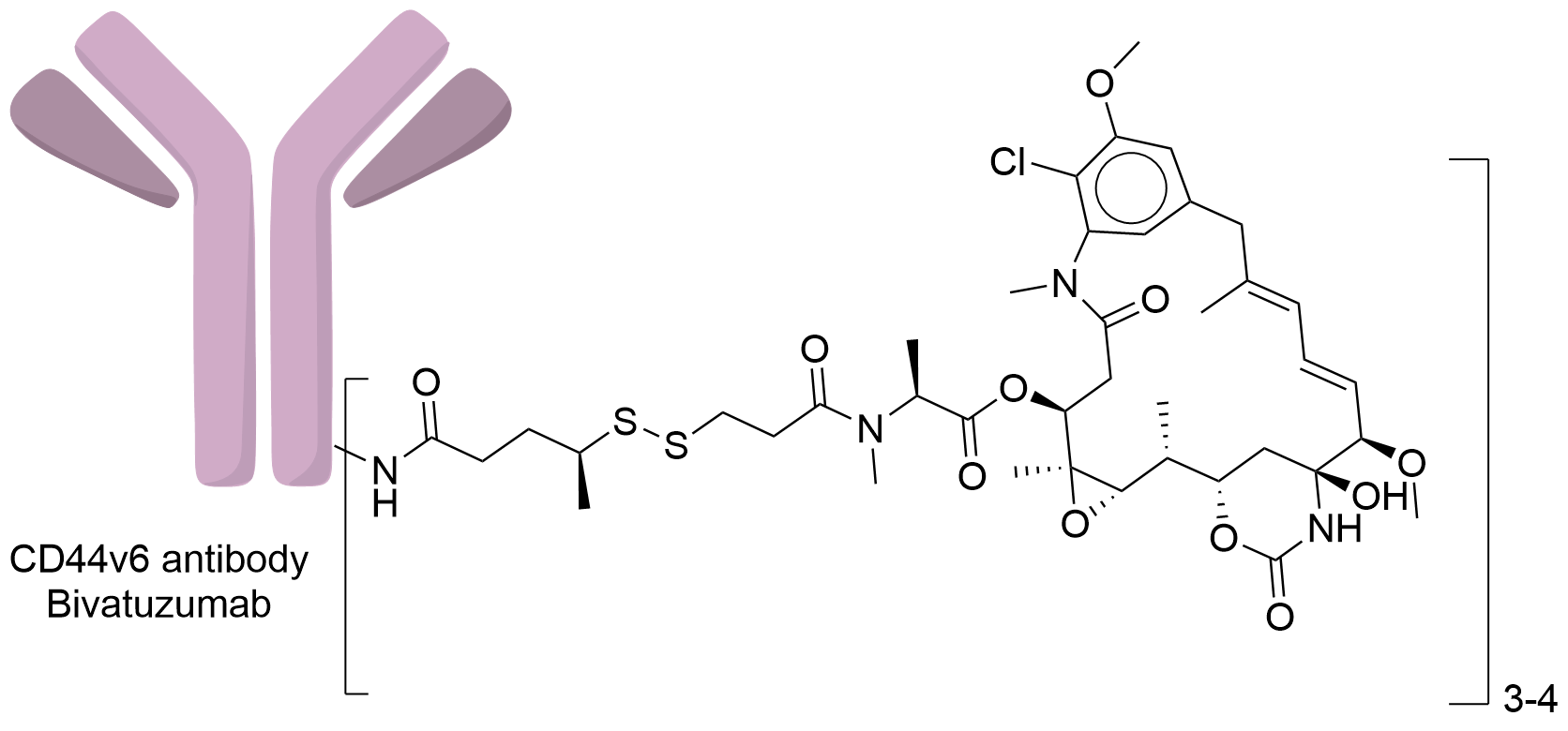
|
|||||
| Antibody Name |
Bivatuzumab
|
Antibody Info | ||||
| Antigen Name |
CD44 antigen (CD44)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM1
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
N-succinimidyl 4-(2-pyridyldithio) pentanoate (SPP)
|
Linker Info | ||||
| Conjugate Type |
Random Lysines
|
|||||
| Combination Type |
mertansine
|
|||||
| Puchem SID | ||||||
| DrugMap ID | ||||||
| TTD ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
0.00%
|
|||
| Patients Enrolled |
Metastatic breast cancer (MBC) that expresses CD44v6 in at least 50% of tumor cells in primary tumor tissue as assessed by immunohistochemistry, pretreatment with anthracyclines and taxanes, tumor metastases measurable by computed tomography (CT) or magnetic resonance imaging (MRI), life expectancy of at least 6 months, no chemotherapy, radiotherapy or immunotherapy within the last 4 weeks before study entry, adequate organ function, Eastern Cooperative Oncology Group performance score 2.
Click to Show/Hide
|
||||
| Administration Dosage |
One single intravenous infusion over 30 min, dose was escalated in 25 mg/m2 increments up to the maximum tolerated dose (MTD).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02254005 | Clinical Status | Phase 1 | ||
| Clinical Description | An open phase 1 single dose escalation study of bivatuzumab mertansine administered intravenously in female patients with CD44v6 positive metastatic breast cancer with repeated administration in patients with clinical benefit. | ||||
| Primary Endpoint |
The MTD in this trial could not be determined.
|
||||
| Other Endpoint |
No objective responses were observed. Disease stabilization was achieved in 50.00% of patients independently of dose level.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02254044 | Clinical Status | Phase 1 | ||
| Clinical Description | An open phase 1 dose escalation study of bivatuzumab mertansine administered intravenously once per week for three weeks in patients with advanced squamous cell carcinoma of the head and neck or esophagus with repeated administration courses in patients with clinical benefit. | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Patients Enrolled |
Ecurrent or metastatic head and neck squamous cell carcinoma (HNSCC) not amenable to established treatments, an ECOG score 2, and an estimated life expectancy of at least 6 months, a tumor diameter of at least 1 cm in CT or MRI scans was also required.
|
||||
| Administration Dosage |
Starting with 25 mg/m2, the dose was escalated in steps of 25 mg/m2 until dose limiting toxicity was observed, intravenously.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02254044 | Clinical Status | Phase 1 | ||
| Clinical Description | An open phase 1 dose escalation study of bivatuzumab mertansine administered intravenously once per week for three weeks in patients with advanced squamous cell carcinoma of the head and neck or esophagus with repeated administration courses in patients with clinical benefit. | ||||
| Primary Endpoint |
The MTD was 300 mg/m2.
|
||||
| Other Endpoint |
Due to the premature discontinuation of the trial efficacy complying with the study plan could not be assessed. In 3 patients,a partial response at doses of 2.00, 2.75 and 3.25 mg/m2.
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02254031 | Clinical Status | Phase 1 | ||
| Clinical Description | An open phase 1 dose escalation study of bivatuzumab mertansine administered intravenously once per week for three weeks in female patients with CD44V6 positive recurrent or metastatic breast cancer with repeated administration courses in patients with clinical. | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02254018 | Clinical Status | Phase 1 | ||
| Clinical Description | An open phase 1 single dose escalation study of bivatuzumab mertansine administered intravenously in patients with advanced squamous cell carcinoma of the head and neck with repeated administration in patients with clinical benefit. | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [6] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02254005 | Clinical Status | Phase 1 | ||
| Clinical Description | An open phase 1 single dose escalation study of bivatuzumab mertansine administered intravenously in female patients with CD44v6 positive metastatic breast cancer with repeated administration in patients with clinical benefit. | ||||
References
