Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0HIGWE
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
CNTO95-DM1
|
|||||
| Synonyms |
CNTO95 DM1
Click to Show/Hide
|
|||||
| Organization |
Sanofi SA
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 3 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3-4
|
|||||
| Structure |
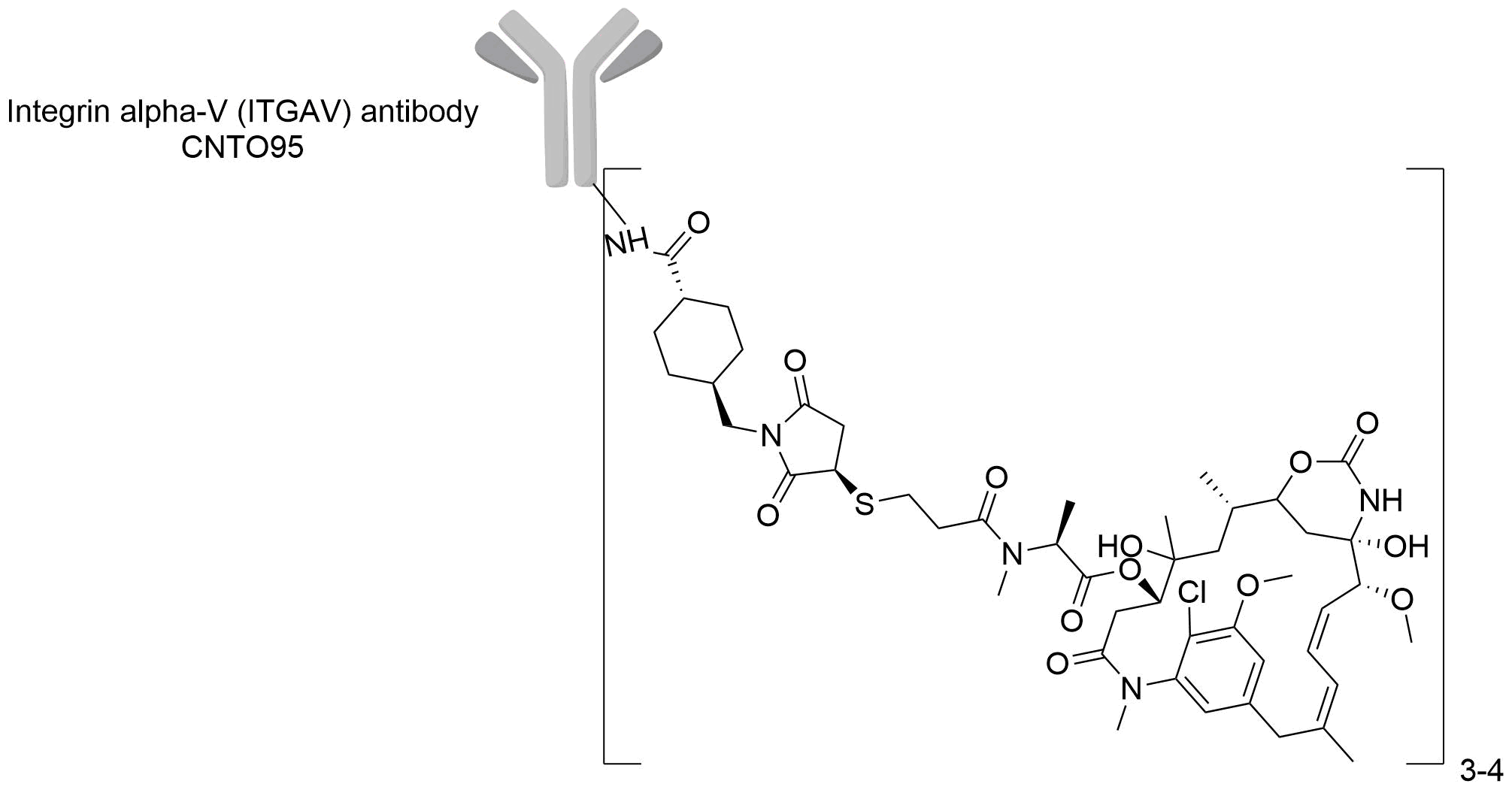
|
|||||
| Antibody Name |
CNTO95
|
Antibody Info | ||||
| Antigen Name |
Integrin alpha-V (ITGAV)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM1
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC)
|
Linker Info | ||||
| Conjugate Type |
Random conjugation through nucleophilic lysines.
|
|||||
| Combination Type |
Emtansine
|
|||||
General Information of The Activity Data Related to This ADC
Discovered Using Cell Line-derived Xenograft Model
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 28.74% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 3 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 44.54% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 6 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.23% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 10 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.81% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 15 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
