Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0QMQKQ
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Trastuzumab-AJICAP-maytansinoid
|
|||||
| Synonyms |
Trastuzumab AJICAP maytansinoid
Click to Show/Hide
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
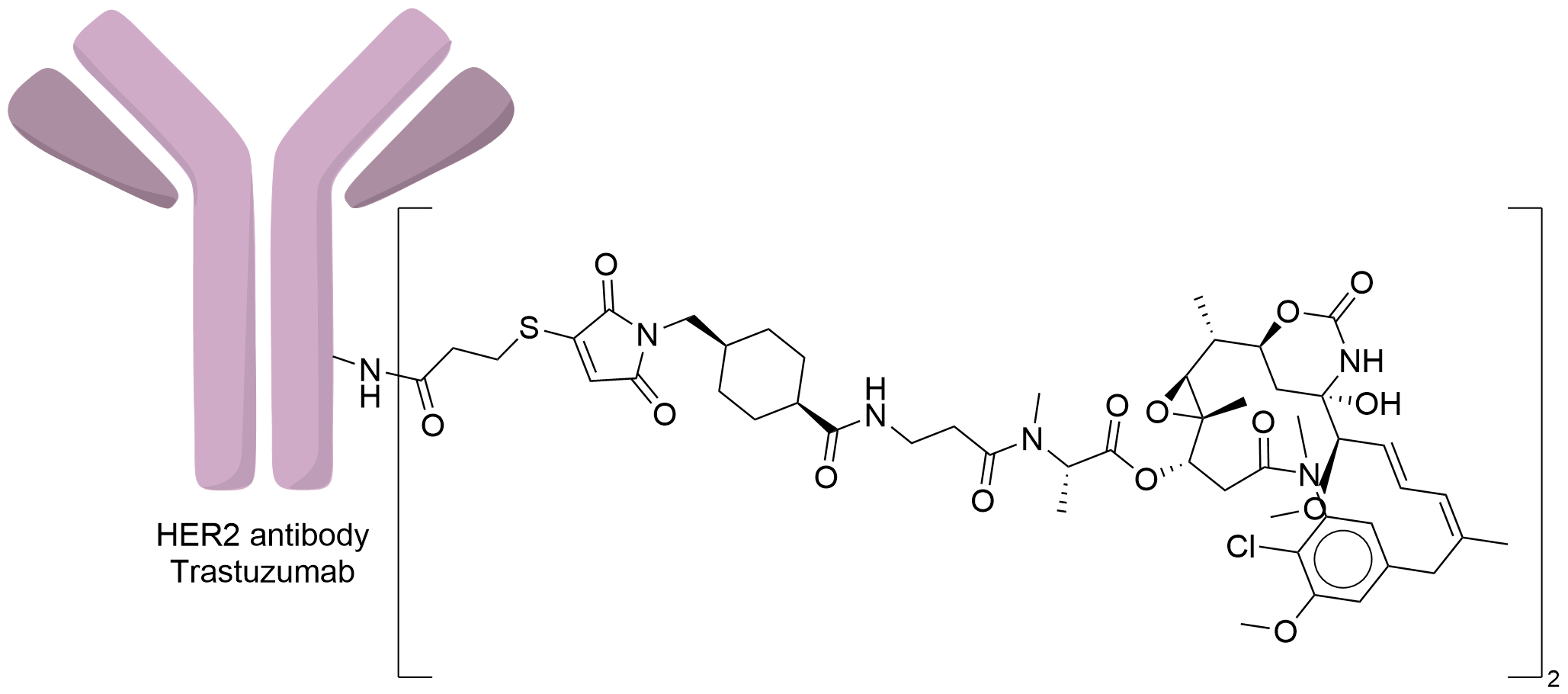
|
|||||
| Antibody Name |
Trastuzumab
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM1
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
AJICAP
|
Linker Info | ||||
| Conjugate Type |
AJICAP site-specific conjugation to synthesize, and ADC conjugation site-occupancy at Lys 248 in the antibody Fc region.
|
|||||
General Information of The Activity Data Related to This ADC
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Minimal Effective Dose (MED) | < 5.00 mg/kg | High HER2 expression (HER2+++) | ||
| Method Description |
Following the acclimatization period (1 week), the animals were stratified by body weight and randomly assigned to the following group: three trastuzumab-AJICAP-maytansinoid groups, treated with 20 mg/kg, 60 mg/kg, and 120 mg/kg. Each group consisted of five animals for blood chemistry test as well as five animals for clinical signs and body weight measurements.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer CDX model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Maximum Tolerated Dose (MTD) | > 120.00 mg/kg | High HER2 expression (HER2+++) | ||
| Method Description |
Following the acclimatization period (1 week), the animals were stratified by body weight and randomly assigned to the following group: three trastuzumab-AJICAP-maytansinoid groups, treated with 20 mg/kg, 60 mg/kg, and 120 mg/kg. Each group consisted of five animals for blood chemistry test as well as five animals for clinical signs and body weight measurements.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer CDX model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
References
