Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0SDZSF
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Lorvotuzumab mertansine
|
|||||
| Synonyms |
BB-10901; IMGN-901; IMGN-901, N901-DM1, BB-10901; IMGN-901-TAP; IMGN901 TAP; Lorvotuzumab mertansine; Monoclonal antibody huN901-DM1 conjugate; huN-901-DM1; huN-901-SPP-DM1; huN901-DM1; huN901-DM1 antibody; huN901-SPP-DM1
Click to Show/Hide
|
|||||
| Organization |
ImmunoGen, Inc.
|
|||||
| Drug Status |
Phase 2 (Terminated)
|
|||||
| Indication |
In total 12 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3 to 4
|
|||||
| Structure |
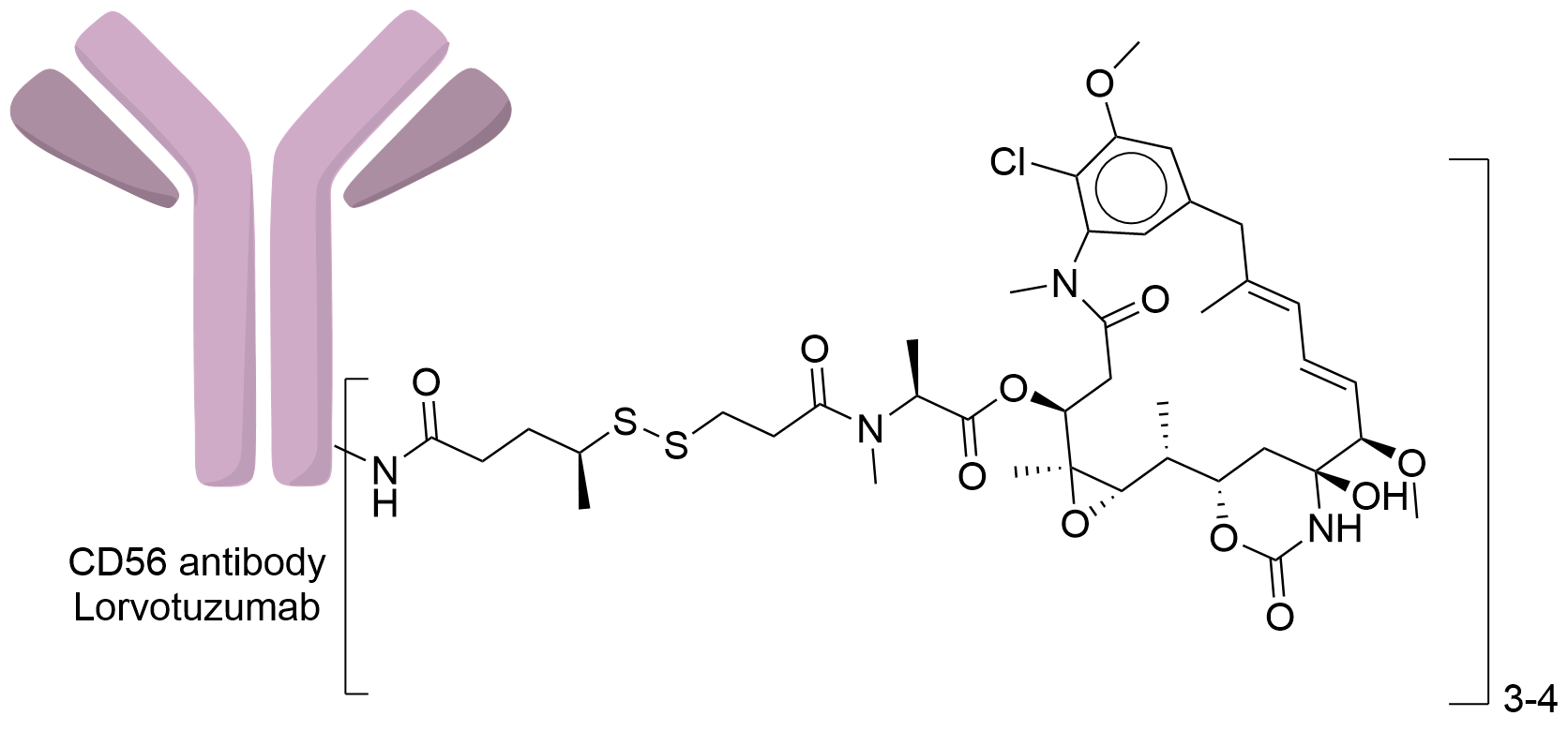
|
|||||
| Antibody Name |
Lorvotuzumab
|
Antibody Info | ||||
| Antigen Name |
Neural cell adhesion molecule 1 (NCAM1)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM1
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
N-succinimidyl 4-(2-pyridyldithio) pentanoate (SPP)
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
mertansine
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 28.30% | Positive CD56 expression (CD56+++/++) | ||
| Patients Enrolled |
Relapsed and/or Refractory CD-56-positive Multiple Myeloma.
|
||||
| Administration Dosage |
40 mg/m2 (up to a maximum of 140 mg) intravenously once every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT00346255 | Clinical Status | Phase 1 | ||
| Clinical Description | BB-10901 in treating patients with relapsed and/or refractory multiple myeloma (IMGN901). | ||||
| Primary Endpoint |
Objective response rate=28.30%.
|
||||
| Other Endpoint |
Median progression-free survival=26.10 months (95% CI 1-89 weeks).
|
||||
References
