Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0HZNEP
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Tmab-SPP-DM1
|
|||||
| Synonyms |
Tmab SPP DM1
Click to Show/Hide
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 3 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3-3.6
|
|||||
| Structure |
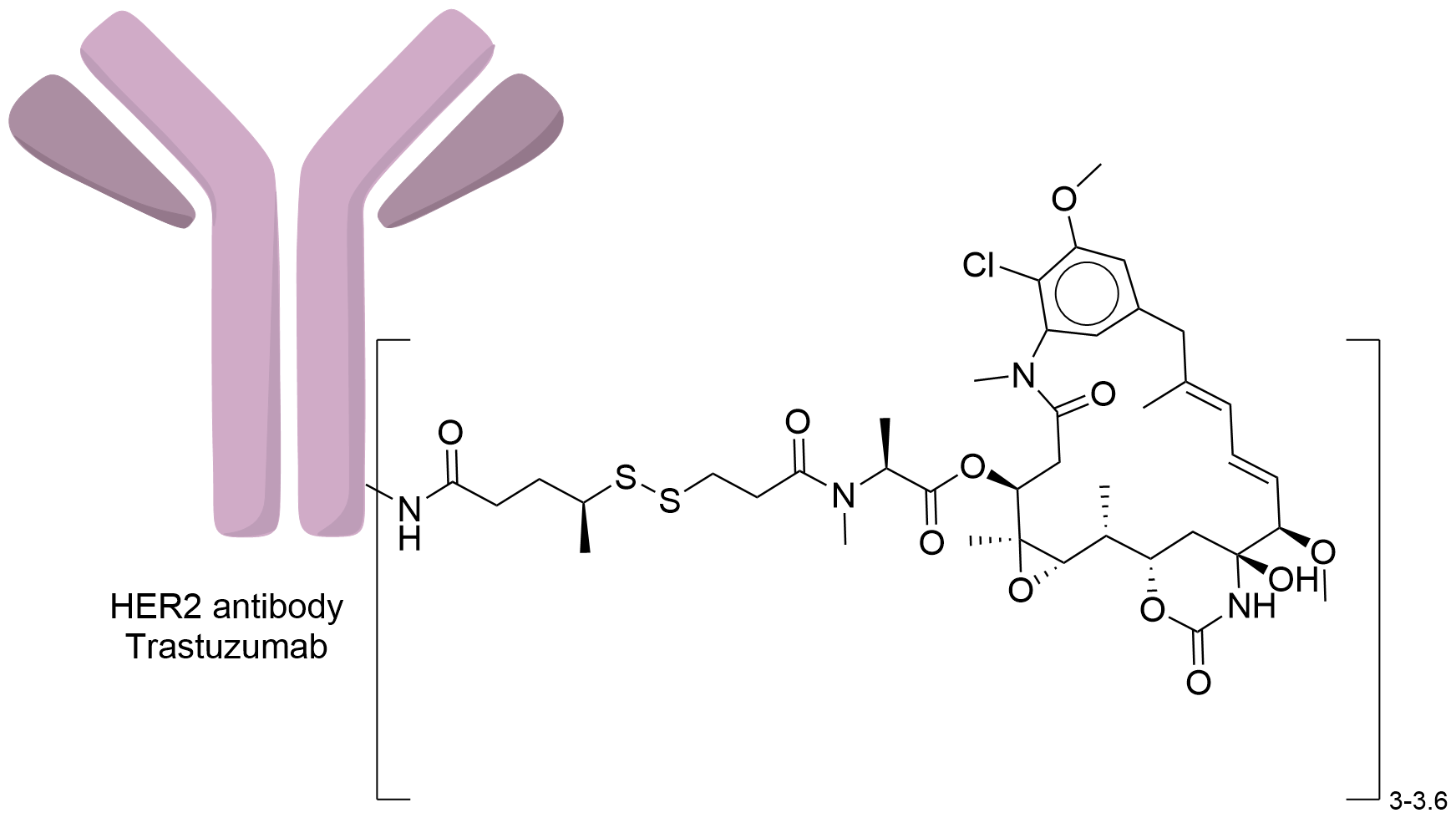
|
|||||
| Antibody Name |
Trastuzumab
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM1
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
N-succinimidyl 4-(2-pyridyldithio) pentanoate (SPP)
|
Linker Info | ||||
| Conjugate Type |
Random conjugating with the free amino group on the antibody.
|
|||||
| Combination Type |
Mertansine
|
|||||
General Information of The Activity Data Related to This ADC
Discovered Using Cell Line-derived Xenograft Model
| Standard Type | Value | Units | Cell Line | Disease Model |
|---|---|---|---|---|
| Tumor Growth Inhibition value (TGI) |
≈ 77.6
|
%
|
MMTV-HER2 cells
|
Breast cancer
|
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.60% (Day 10) | High HER2 expression (HER2+++) | ||
| Method Description |
Mice bearing mammary tumor transplants from the MMTV-HER2 Fo5 line were given a single iv injection (10 mg/kg) of Tmab-SPP-DM1, Tmab-SSNPP-DM3, Tmab-SSNPP-DM4, Tmab-MCC-DM1, or vehicle (n=7 mice per group), and tumor growth was monitored for 25 days.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.22 nM | High HER2 expression (HER2+++) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. For measurement of apoptosis, BT-474 and SK-BR-3 were exposed to trastuzumab or trastuzumab-DM for 48 h.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 4.26 nM | High HER2 expression (HER2+++) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. For measurement of apoptosis, BT-474 and SK-BR-3 were exposed to trastuzumab or trastuzumab-DM for 48 h.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 41.37 nM | Negative HER2 expression (HER2-) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100.00 nM | Low HER2 expression (HER2+) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
References
