Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0DIWSF
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Cantuzumab mertansine
|
|||||
| Synonyms |
C-242 DM1; C-242 May; C242 maytansinoid conjugate; Cantuzumab mertansine; Monoclonal antibody C-242 DM1 conjugate; Monoclonal antibody C-242 May conjugate; Monoclonal antibody huC242-May conjugate; SB-408075; huC242 maytansinoid conjugate; huC242-DM1
Click to Show/Hide
|
|||||
| Organization |
ImmunoGen, Inc.
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
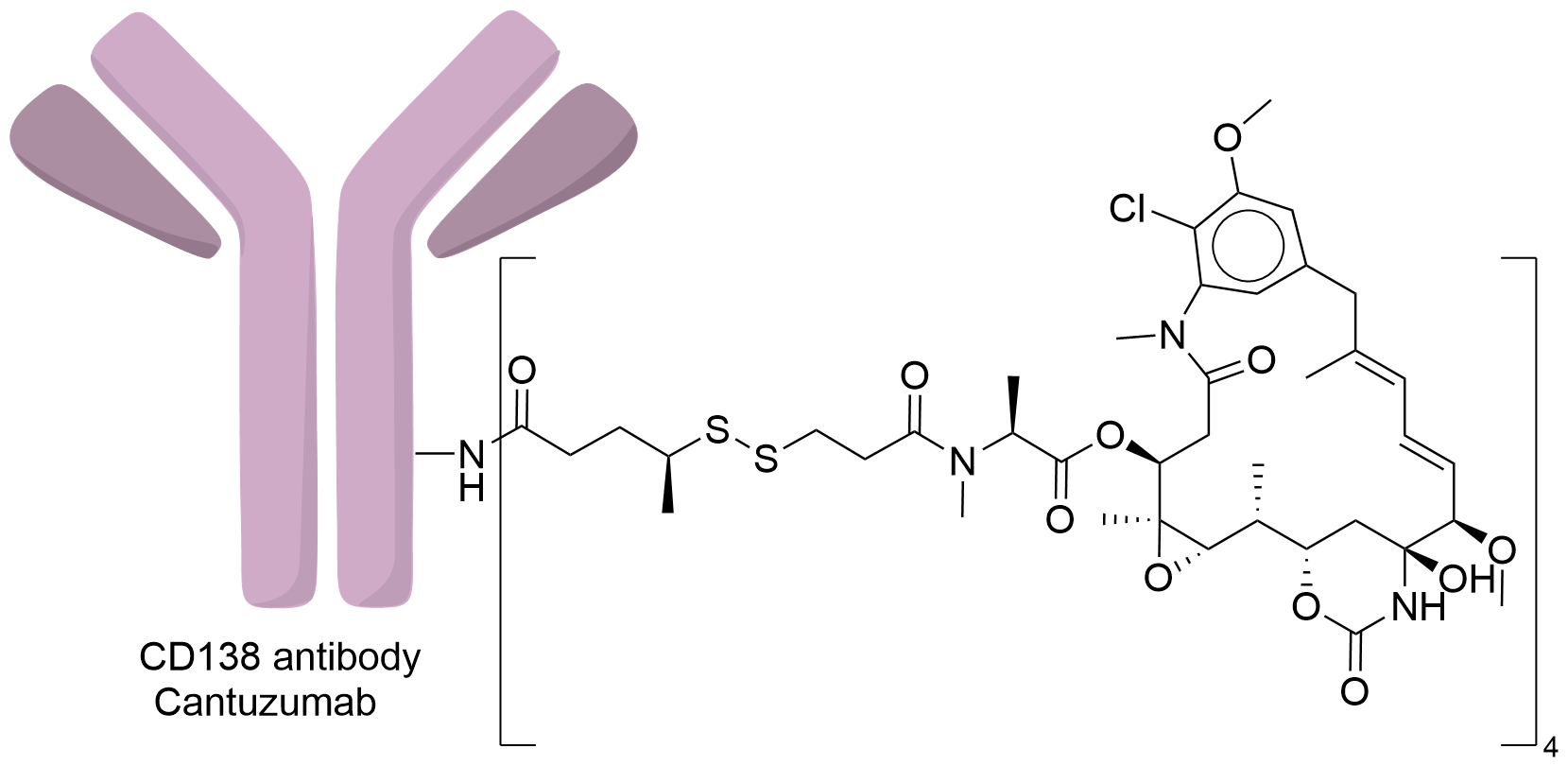
|
|||||
| Antibody Name |
Cantuzumab
|
Antibody Info | ||||
| Antigen Name |
Mucin-1 (MUC1)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM1
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
N-succinimidyl 4-(2-pyridyldithio) pentanoate (SPP)
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
mertansine
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| TTD ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
0.00%
|
|||
| Patients Enrolled |
Histological documentation of advanced or metastatic epithelial solid tumor which were likely to express the CanAg antigen, that were refractory or resistant to standard chemotherapy, or for which no effective standard therapy exists.
|
||||
| Administration Dosage |
IV infusion at an initial dose of 30 mg/m2, three times per week for three consecutive weeks for a total of nine doses.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Patients Enrolled |
Solid malignancies refractory to standard therapy or for whom no standard therapy existed.
|
||||
| Administration Dosage |
IV cantuzumab mertansine was administered at a rate of 1 mg/min for 30 minutes and then increased to 3 mg/min if hypersensitivity phenomena were not observed. Treatment courses were repeated every 3 weeks.
|
||||
References
