Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0RYBMQ
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
SHR-A1201
|
|||||
| Synonyms |
SHR A1201; SHR-A1201; SHRA1201
Click to Show/Hide
|
|||||
| Organization |
Jiangsu Hengrui Pharmaceuticals Co., Ltd.; Shanghai Hengrui Pharmaceutical Co., Ltd.
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3.5
|
|||||
| Structure |
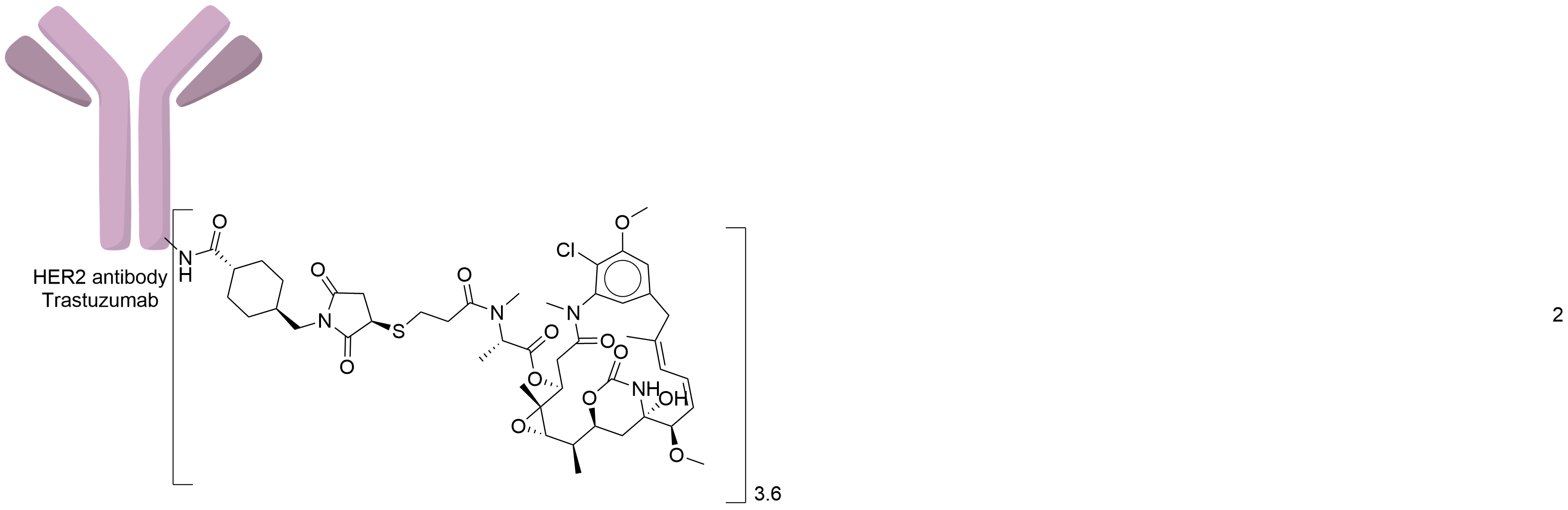
|
|||||
| Antibody Name |
Trastuzumab
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM1
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC)
|
Linker Info | ||||
| Conjugate Type |
Random Lysines
|
|||||
| Combination Type |
emtansine
|
|||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Patients Enrolled |
HER2-positive locally advanced or metastatic breast cancer (positivity for HER2 was defined as a score of 2+ in immunohistochemical analysis and a positive result in fluorescence in situ hybridization or a score of 3+ in immunohistochemical analysis), the standard treatment was invalid or there was no effective standard treatment plan, the Eastern Cooperative Oncology Group performance status score was 0-1.
Click to Show/Hide
|
||||
| Administration Dosage |
4 dose-escalation sequences, 1.20 mg/kg, 2.40 mg/kg, 3.60 mg/kg and 4.80 mg/kg, once every 21days, as a 90-min intravenous infusion.
|
||||
References
