Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0FNCKV
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
HuFR1-65-SMCC-DM1
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 4 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3.8
|
|||||
| Structure |
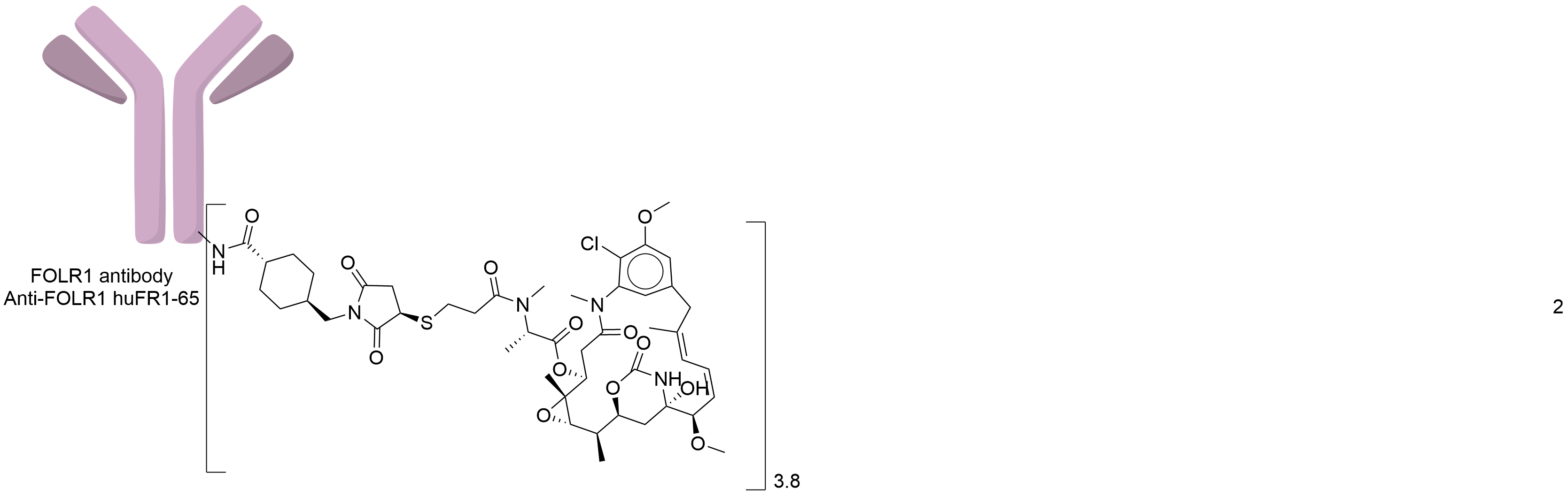
|
|||||
| Antibody Name |
Anti-FOLR1 huFR1-65
|
Antibody Info | ||||
| Antigen Name |
Folate receptor alpha (FOLR1)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM1
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC)
|
Linker Info | ||||
| Conjugate Type |
Random conjugation through nucleophilic lysines.
|
|||||
| Combination Type |
Emtansine
|
|||||
General Information of The Activity Data Related to This ADC
Discovered Using Cell Line-derived Xenograft Model
| Standard Type | Value | Units | Cell Line | Disease Model |
|---|---|---|---|---|
| Tumor Growth Inhibition value (TGI) |
65
|
%
|
KB cells
|
Human papillomavirus-related endocervical adenocarcinoma
|
Revealed Based on the Cell Line Data
| Standard Type | Value | Units | Cell Line | Disease Model |
|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) |
0.08±0.02
|
nM
|
KB cells
|
Human papillomavirus-related endocervical adenocarcinoma
|
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 65.00% (Day 12) | High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Five-week-old female CB-17 severe combined immunodeficient (SCID) mice were obtained and quarantined for 7 days prior to study initiation Mice were inoculated subcutaneously with KB cells (1 x107 cells per mouse) resuspended in serum-free culture mediaMice with established KB xenografts were dosed with a single intravenous injection of 200 g of conjugated maytansinoid per kg, equivalent to 102 mg/kg antibody on day 6 after cell inoculationTumor dimensions were measured twice weekly and volume was calculated as (A B2) 05, where A represents the largest and B the perpendicular tumor diameter.
Click to Show/Hide
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.08±0.02 nM | High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
References
