Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0BFNMH
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
GQ-1001
|
|||||
| Synonyms |
GQ 1001; GQ-1001; GQ1001; GQ1001(Trastuzumab-ADC)
Click to Show/Hide
|
|||||
| Organization |
GeneQuantum Healthcare (Suzhou) Co. Ltd.
|
|||||
| Drug Status |
Phase 1
|
|||||
| Indication |
In total 4 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
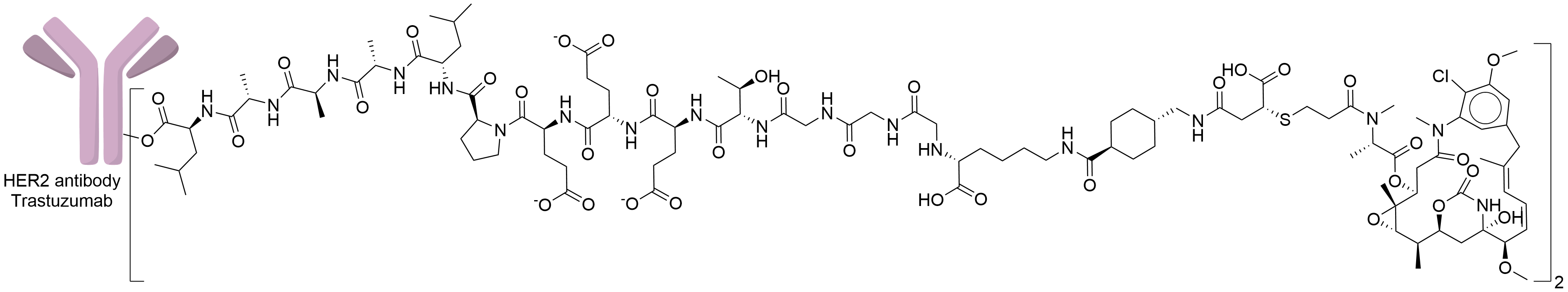
|
|||||
| Antibody Name |
Trastuzumab
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM1
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
MCC-Gly6-Thr-Glu3-Pro-Leu-Ala3-Leu
|
Linker Info | ||||
| Conjugate Type |
Enzymatic Catalysis
|
|||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Partial Response (PR) |
40.00%
|
|||
| Patients Enrolled |
HER2-positive advanced solid tumors.
|
||||
| Administration Dosage |
Administered intravenously as a monotherapy on Day 1 of 21-day cycles. The starting dose was 1.20 mg/kg, followed by 2.40, 3.60, 4.80, 6.00, 7.20 and 8.40 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT04450732 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, first-in-human, multicenter, open-label, study of GQ1001, a HER2 targeted antibody-drug conjugate, administered intravenously, in adult patients with HER2-positive advanced solid tumors. | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05575804 | Clinical Status | Phase 1/2 | ||
| Clinical Description | Phase 1b/2 study of GQ1001 and pyrotinib in HER2 positive metastatic breast cancer patients who had failed previous anti-HER2 treatment GRACE. | ||||
References
