Linker Information
General Information of This Linker
| Linker ID |
LIN0YRUBS
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
Mal-PEG8-Val-Ala-PABC
|
|||||
| Linker Type |
Cathepsin-cleavable linker
|
|||||
| Antibody-Linker Relation |
Cleavable
|
|||||
| Structure |
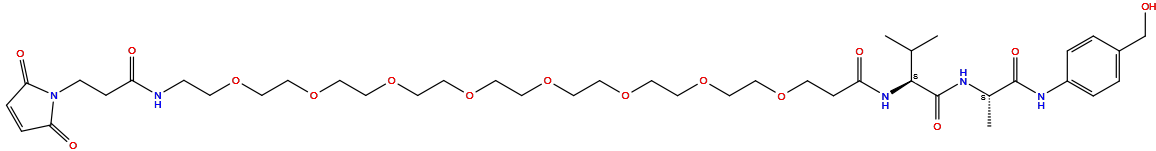
|
|||||
| Formula |
C41H65N5O15
|
|||||
| Isosmiles |
[H]OC([H])([H])c1c([H])c([H])c(N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N([H])C(=O)C([H])([H])C([H])([H])OC([H])([H])C([H])([H])OC([H])([H])C([H])([H])OC([H])([H])C([H])([H])OC([H])([H])C([H])([H])OC([H])([H])C([H])([H])OC([H])([H])C([H])([H])OC([H])([H])C([H])([H])OC([H])([H])C([H])([H])N([H])C(=O)C([H])([H])C([H])([H])N2C(=O)C([H])=C([H])C2=O)C([H])(C([H])([H])[H])C([H])([H])[H])C([H])([H])[H])c([H])c1[H]
|
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C41H65N5O15/c1-31(2)39(41(53)43-32(3)40(52)44-34-6-4-33(30-47)5-7-34)45-36(49)11-14-54-16-18-56-20-22-58-24-26-60-28-29-61-27-25-59-23-21-57-19-17-55-15-12-42-35(48)10-13-46-37(50)8-9-38(46)51/h4-9,31-32,39,47H,10-30H2,1-3H3,(H,42,48)(H,43,53)(H,44,52)(H,45,49)/t32-,39-/m0/s1
|
|||||
| InChIKey |
YJYDWLGCCNOUSJ-GSSDHLSPSA-N
|
|||||
| IUPAC Name |
(2S)-2-[3-[2-[2-[2-[2-[2-[2-[2-[2-[3-(2,5-dioxopyrrol-1-yl)propanoylamino]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]propanoylamino]-N-[(2S)-1-[4-(hydroxymethyl)anilino]-1-oxopropan-2-yl]-3-methylbutanamide
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
867.991
|
Polar area
|
247.85
|
||
|
Complexity
|
867.4477164
|
xlogp Value
|
-0.2829
|
|||
|
Heavy Count
|
61
|
Rot Bonds
|
41
|
|||
|
Hbond acc
|
15
|
Hbond Donor
|
5
|
|||
Each Antibody-drug Conjugate Related to This Linker
Full Information of The Activity Data of The ADC(s) Related to This Linker
Loncastuximab tesirine [Approved]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
48.28%
|
|||
| Patients Enrolled |
Relapsed or refractory diffuse large B-cell lymphoma (DLBCL) after two or more multiagent systemic treatments, who had measurable disease and Eastern Cooperative Oncology Group performance status 0-2.
|
||||
| Administration Dosage |
Intravenously on day 1 of each 21-day cycle, at 150 ug/kg for two cycles, then 75 ug/kg thereafter, for up to 1 year or until disease relapse or progression, unacceptable toxicity, death, major protocol deviation, pregnancy, or patient, investigator, or sponsor decision.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03589469 | Clinical Status | Phase 2 | ||
| Clinical Description |
A phase 2 open-label single-arm study to evaluate the efficacy and safety of loncastuximab tesirine in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) (LOTIS-2).
|
||||
| Primary Endpoint |
Overall response rate assessed by central review. 70 of 145 patients had complete or partial response (overall response rate 48.28% [95% CI 39.9-56.7]); 35 had complete response and 35 had partial response.
|
||||
| Other Endpoint |
Median time to first response (complete response or partial response) was 41.00 days (IQR 38.00-44.00). Median duration of response was 10.30 months (95% CI 6.9-not estimable); 13.40 months (10.30-not estimable) for patients with complete response and 5.70 months (1.70-not estimable) for patients with partial response. The probability of responders maintaining responses for 9 months or longer was 64.0%. Median progressionfree survival was 4.90 months (95% CI 2.90-8.30), median overall survival was 9.90 months (6.70-11.50), and median relapse-free survival was 13.40 months (10.30-not estimable).
Click to Show/Hide
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
59.40%
|
|||
| Patients Enrolled |
R/R B-cell non Hodgkin lymphoma (NHL); had failed or were intolerant to established therapies, or for whom no other established treatment options were available.
|
||||
| Administration Dosage |
5 to 200 ug/kg; intravenously over 1 hour, once every 3 weeks (one cycle) by a 3+3 dose-escalation design.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02669017 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1 dose-escalation study to evaluate the tolerability, safety, pharmacokinetics, and antitumor activity of ADCT-402 in patients with relapsed or refractory b-cell lineage non Hodgkin lymphoma (B-NHL).
|
||||
| Primary Endpoint |
The MTD was not established during the trial due to the low level of DLTs, 150 ug/kg dose for expansion and phase 2.
|
||||
| Other Endpoint |
For loncastuximab tesirine 120 ug/kg,Median Progression-Free Survival (mPFS)=4.80 months.The median PFS OS=11.60 months.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Complete Remission (CR) |
26.70%
|
|||
| Patients Enrolled |
Adults (age 18 years) with histologically confirmed relapsed/refractory B-non Hodgkin lymphoma (World Health Organization 2008 classification18 ) who were intolerant to established therapy, for whom established therapy had failed, or for whom no other treatment options were available in the opinion of the investigator were eligible to participate.
|
||||
| Administration Dosage |
3 + 3 dose escalation at 15 to 200 ug/kg and dose expansion at 120 and 150 ug/kg; IV infusion over 60 minutes once every 3 weeks (day 1 of each 21-day cycle).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02669017 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1 dose-escalation study to evaluate the tolerability, safety, pharmacokinetics, and antitumor activity of ADCT-402 in patients with relapsed or refractory B-cell lineage non Hodgkin lymphoma (B-NHL).
|
||||
| Primary Endpoint |
The recommended dose of loncastuximab tesirine for Phase 2 is 150 ug/kg Q3W for 2 doses followed by 75 ug/kg Q3W for subsequent doses. The 150 ug/kg dose was selected as a dose with encouraging responses but lower frequency of AEs than observed with the 200 u/kg dose. Overall response rate (ORR) in evaluable patients was 45.60%, including 26.70% complete responses (CR). Median PFS was 3.10 months (95% CI 2.70-4.20) in all patients with B-NHL, 2.8 months (95% CI 1.90-3.80) in patients with DLBCL, 4.80 months (95% CI 1.10-7.80) in patients with MCL, and could not be determined in those with FL due to the low number of events. Median OS was 8.3 months (95% CI 6.70-10.70) in all patients with B-NHL, 7.5 months (95% CI 6.00-9.80) in patients with DLBCL, and was not reached in patients with MCL or FL due to low number of events.
Click to Show/Hide
|
||||
| Other Endpoint |
PK exposure similarity between loncastuximab tesirine total antibody and PBD-conjugated antibody indicated good stability in serum.Accumulation by Cycle 2 for patients on a Q6W dosing regimen was lower than that of those on Q3W dosing: mean accumulation of 1.22 and 1.33 for PBD-conjugated antibody and total antibody on Q6W regimens compared with 1.72 and 1.74 on Q3W regimens. There was substantial variability in PK exposure and PK parameters assessed for PBD-conjugated antibody, total antibody, and SG3199.
Click to Show/Hide
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Complete Remission (CR) |
40.20%
|
|||
| Patients Enrolled |
R/R B-acute lymphocytic leukemia (ALL) for standard therapies had failed, intolerant to standard therapies, or no other treatment options were available, in the opinion of the investigator, were eligible for the study, other key inclusion criteria included Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2.
|
||||
| Administration Dosage |
15 to 150 ug/kg once every 3 weeks (Q3W) or 50 ug/kg IV weekly.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02669264 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1, open-label, adaptive dose-escalation, multicenter study to evaluate the tolerability, safety, pharmacokinetics, and anti-tumor activity of ADCT-402 in patients with relapsed or refractory B-cell lineage acute lymphoblastic leukemia (B-ALL).
|
||||
| Primary Endpoint |
Part II (dose expansion portion) utilized the doses of 120 and 150 ug/kg, selected based on antitumor activity and tolerability seen during part I. The overall response rate (ORR) in patients with DLBCL, mantle cell lymphoma, and follicular lymphoma was 42.30%, 46.70%, and 78.60%, respectively.
|
||||
| Other Endpoint |
The median progression-free survival (PFS) was 3.10 months in all patients with B-NHL and 2.80 months in patients with DLBCL, whereas the median OS was 8.30 months in all patients and 7.50 months in patients with DLBCL.
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Complete Remission (CR) |
46.16%
|
|||
Rovalpituzumab tesirine [Phase 3 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
10.00%
|
|||
| Patients Enrolled |
Extensive-stage small-cell lung cancer (ES-SCLC) who had completed four cycles of front-line platinum-based chemotherapy (cisplatin or carboplatin with etoposide or irinotecan) at least 3 weeks but not more than 9 weeks before randomization and had stable disease, PR, or CR per RECIST v.1.1.
|
||||
| Administration Dosage |
0.30 mg/kg intravenous Rova-T on day 1 of each 6-week cycle, omitting every third cycle.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03033511 | Clinical Status | Phase 3 | ||
| Clinical Description |
A randomized, double-blind, placebo-controlled phase 3 study of rovalpituzumab tesirine as maintenance therapy following first-line platinum-based chemotherapy in subjects with extensive stage small cell lung cancer (MERU).
|
||||
| Primary Endpoint |
Median age of all randomized patients (N=748) was 64 years; 78.00% had TNM stage IV disease. At futility analysis of the subset with DLL3-high tumors, the hazard ratio for OS was 1.07 (95% confidence interval: 0.84-1.36) favoring the placebo arm,with median OS of 8.50 and 9.80 months in the Rova-T and placebo arms,respectively; futility criteria were met. Rova-T significantly improved PFS versus placebo by investigator assessment (4.00 versus 1.40 mo,hazard ratio=0.48, p < 0.001).
Click to Show/Hide
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
12.40% (all)
14.30% (DLL3-high) 13.20% (DLL3-positive) |
|||
| Patients Enrolled |
Advanced stage DLL3-positive small-cell lung cancer (SCLC).
|
||||
| Administration Dosage |
0.30 mg/kg Rova-T intravenously infused over 30 minutes once every 6 weeks for two cycles.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02674568 | Clinical Status | Phase 2 | ||
| Clinical Description |
An Open-label, Single-Arm, Phase 2 Study Evaluating the Efficacy, Safety and Pharmacokinetics of Rovalpituzumab Tesirine (SC16LD6.5) for Third-line and Later Treatment of Subjects With Relapsed or Refractory Delta-Like Protein 3-Expressing Small Cell Lung Cancer (TRINITY).
|
||||
| Primary Endpoint |
OrR was 12.40%, 14.30%, and 13.20% in all, DLL3-high, and DLL3-positive patients,respectively. Median OS was 5.60 months in all patients and 5.70 months in DLL3-high patients.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
17.14% (In pooled patients with NEC/NET expressing a high level of DLL 3% (50% DLL3-positive tumor cells))
8.82% (In those with NEC/NET expressing a low level of DLL 3% (1-49% DLL3-positive tumor cells)) |
|||
| Patients Enrolled |
101 with NEC/NET (large-cell NEC, gastroenteropancreatic NEC, neuroendocrine prostate cancer, and other NEC/NET) and 99 with other solid tumors (melanoma, medullary thyroid cancer [MTC], glioblastoma, and other).
|
||||
| Administration Dosage |
The recommended phase II dose (RP2D) was 0.30 mg/kg every 6 weeks (q6w) for two cycles.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02709889 | Clinical Status | Phase 1/2 | ||
| Clinical Description |
An open-label study of rovalpituzumab tesirine in subjects with delta-like protein 3-expressing advanced solid tumors.
|
||||
| Primary Endpoint |
The recommended phase II dose (RP2D) was 0.30 mg/kg every 6 weeks (q6w) for two cycles. At the RP2D, grade 3/4 adverse events included anemia (17.00%), thrombocytopenia (15.00%), and elevated aspartate aminotransferase (8.00%). Responses were confirmed in 15/145 patients (10.34%) treated at 0.30 mg/kg, including 9/69 patients (13.04%) with NEC/NET. Rova-T at 0.30 mg/kg q6w had manageable toxicity, with antitumor activity observed in patients with NEC/NET, melanoma, MTC, and glioblastoma.
Click to Show/Hide
|
||||
| Other Endpoint |
In pooled patients with NEC/NET expressing a high level of DLL3 (50% DLL3-positive tumor cells), the ORR was 17.14% (6/35) and 34.29% (12/35) had a BOR (all PRs). In those with NEC/NET expressing a low level of DLL3 (1-49% DLL3-positive tumor cells), the ORR was 8.82% (3/34) and the BOR rate was 14.70% (5/34) (all PRs). The median PFS values for pooled patients with NEC/NET expressing high and low levels of DLL3 were 4.30 months (95% CI, 2.7-6.1) and 3.30 months (95% CI, 2.40-4.80), respectively. The median OS values for patients expressing high and low levels of DLL3 were 7.40 months (95% CI, 5.60-13.10) and 7.10 months (95% CI, 4.30-9.90), respectively.
Click to Show/Hide
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
34.50% (All evaluable patients)
31.60% (DLL3 score 75%) 50.00% (PD-L1 positive tumor cells) 50.00% (PD-L1 positive inflammatory cells with an intensity of 2+) |
|||
| Patients Enrolled |
Progressive small-cell lung cancer (SCLC) who had previously been treated with at least one prior line of platinum-containing chemotherapy were enrolled if they were naive to PD-1/PD-L1targeting agents, had ECOG performance status 0-1, measurable disease per RECIST v1.1 or disease evaluable by tumor antigen assessment, and adequate bone marrow, cardiac, hepatic, and renal functions.
Click to Show/Hide
|
||||
| Administration Dosage |
Budigalimab 375 mg via intravenous infusion every 3 weeks and Rova-T was administered as a dose of 0.30 mg/kg intravenously, on day 1 of the first and third 3-week cycle.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03000257 | Clinical Status | Phase 1 | ||
| Clinical Description |
A multicenter, phase 1, open-label, dose-escalation study of ABBV-181 as monotherapy and in combination with another anti-cancer therapy in subjects with advanced solid tumors.
|
||||
| Primary Endpoint |
In patients with DLL3 score 75.00% (n = 19) the response rate was similar to the total evaluable population, with an ORR of 21.10% (90% CI: 7.50-41.90).
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
50.00% (all)
63.00% (0.1 mg/kg Rova-T) 33.00% (0.2 mg/kg Rova-T) |
|||
| Patients Enrolled |
Extensive-stage small-cell lung cancer (ES SCLC), with a response of stable disease or better after the prestudy CE cycle per the Response Evaluation Criteria in Solid Tumors version 1.1, Eastern Cooperative Oncology Group performance status of 0 to 1, and absent or treated central nervous system metastases.
|
||||
| Administration Dosage |
Rova-T monotherapy (0.30 mg/kg, every 6 [q6] wk 2; cohort 1; n = 4); Rova-T induction (0.30 mg/kg, q6 wk 2) followed by CE every 21 days (q21) 4 (cohort 2; n = 5); Rova-T (0.10 or 0.20 mg/kg, q6 wk 2) overlapping with CE q21 4 (cohort 3; n = 14); and Rova-T maintenance (0.30 mg/kg, q6 wk 2) after CE q21 4 (cohort 4; n = 3).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02819999 | Clinical Status | Phase 1 | ||
| Clinical Description |
A study of rovalpituzumab tesirine (SC16LD6.5) in the frontline treatment of patients with extensive stage small cell lung cancer.
|
||||
| Primary Endpoint |
Median age was 66 years, and 73.00% had Eastern Cooperative Oncology Group performance status of 1. In cohort 3,seven patients (50%) had confirmed objective responses,with a median progression-free survival of 5.20 months and median overall survival of 10.30 months.
|
||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 16.00% (Day 9) | High DLL3 expression (DLL3+++) | ||
| Method Description |
The inhibitory activity of Rova-T against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.1 mg/kg.
|
||||
| In Vivo Model | Neuroblastoma PDX model (PDX: COG-N-415x) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 30.00% (Day 9) | High DLL3 expression (DLL3+++) | ||
| Method Description |
The inhibitory activity of Rova-T against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.3 mg/kg.
|
||||
| In Vivo Model | Neuroblastoma PDX model (PDX: COG-N-415x) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 33.00% (Day 28) | High DLL3 expression (DLL3+++) | ||
| Method Description |
The inhibitory activity of Rova-T against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 1 mg/kg weekly x 1.
|
||||
| In Vivo Model | Neuroblastoma PDX model (PDX: COG-N-415x) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 40.74% (Day 9) | High DLL3 expression (DLL3+++) | ||
| Method Description |
The inhibitory activity of Rova-T against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.1 mg/kg.
|
||||
| In Vivo Model | Neuroblastoma PDX model (PDX: COG-N-452x) | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 50.90% (Day 10) | High DLL3 expression (DLL3+++) | ||
| Method Description |
The inhibitory activity of Rova-T against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.1 mg/kg.
|
||||
| In Vivo Model | Neuroblastoma PDX model (PDX: COG-N-519x) | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 59.81% (Day 9) | High DLL3 expression (DLL3+++) | ||
| Method Description |
The inhibitory activity of Rova-T against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.3 mg/kg.
|
||||
| In Vivo Model | Neuroblastoma PDX model (PDX: COG-N-452x) | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 73.87% (Day 10) | High DLL3 expression (DLL3+++) | ||
| Method Description |
The inhibitory activity of Rova-T against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.3 mg/kg.
|
||||
| In Vivo Model | Neuroblastoma PDX model (PDX: COG-N-519x) | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 78.50% (Day 9) | High DLL3 expression (DLL3+++) | ||
| Method Description |
The inhibitory activity of Rova-T against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.6 mg/kg.
|
||||
| In Vivo Model | Neuroblastoma PDX model (PDX: COG-N-452x) | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 83.00% (Day 9) | High DLL3 expression (DLL3+++) | ||
| Method Description |
The inhibitory activity of Rova-T against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.6 mg/kg.
|
||||
| In Vivo Model | Neuroblastoma PDX model (PDX: COG-N-415x) | ||||
| Experiment 10 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.14% (Day 10) | High DLL3 expression (DLL3+++) | ||
| Method Description |
The inhibitory activity of Rova-T against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.6 mg/kg.
|
||||
| In Vivo Model | Neuroblastoma PDX model (PDX: COG-N-519x) | ||||
| Experiment 11 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.83% (Day 10) | High DLL3 expression (DLL3+++) | ||
| Method Description |
The inhibitory activity of Rova-T against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.6 mg/kg.
|
||||
| In Vivo Model | Neuroblastoma PDX model | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.00% (Day 28) | High DLL3 expression (DLL3+++) | ||
| Method Description |
The inhibitory activity of Rova-T against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 1 mg/kg weekly x 3.
|
||||
| In Vivo Model | COG-N-415 neuroblastoma model | ||||
| In Vitro Model | Neuroblastoma | COG-N-415 cells | CVCL_AQ23 | ||
Camidanlumab tesirine [Phase 2]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Complete Remission (CR) |
48.60% (In the cHL cohort, at 45 ug/kg)
35.00% (In the cHL cohort, at 30 ug/kg) 6.50% (in all patients with T-NHL) 10.00% (in all patients with T-NHL, 60 ug/kg doses) 7.10% (in all patients with T-NHL, 80 ug/kg doses) 0.00% (in all patients with T-NHL, 100 ug/kg doses) |
|||
| Patients Enrolled |
Relapsed or refractory classical Hodgkin lymphoma or non-Hodgkin lymphoma, an Eastern Cooperative Oncology Group performance status 0-2, who had no therapies available to them with established clinical benefit for their disease stage were enrolled.
|
||||
| Administration Dosage |
Intravenously (3-150 ug/kg) once every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02432235 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1 adaptive dose-escalation study to evaluate the tolerability, safety, pharmacokinetics, and antitumor activity of ADCT-301 in patients with relapsed or refractory Hodgkin lymphoma and non-Hodgkin lymphoma.
|
||||
| Primary Endpoint |
The maximum tolerated dose was not reached. The recommended doses for expansion were 30 ug/kg and 45 ug/kg for patients with classical Hodgkin lymphoma and 80 ug/kg for patients with T-cell non-Hodgkin lymphomas. No recommended doses for expansion were defined for B-cell non-Hodgkin lymphomas.
|
||||
| Other Endpoint |
In the cHL cohort,ORR (95% CI) was 86.49% at 45 ug/kg (32/37 patients [71.20-95.50]) and 55.00% at 30 ug/kg (11/20 patients [31.50-76.90]); 48.65% (18/37 patients) and 35.00% (7/20 patients) achieved CR. Median (95% CI) DOR for the cHL population was 6.64 months (5.06-8.11),and was 7.16 months (4.57-8.51) in patients treated at 45 ug/kg.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [13] | ||||
| Patients Enrolled |
Antecedent myelodysplastic syndrome who received treatment with hypomethylating agents and subsequently presented with CD25-positive AmL, or patients with R/R CD25-positive acute lymphocytic leukemia (ALL) who had failed or were intolerant to any established therapy, or for whom no other treatment options were available.
|
||||
| Administration Dosage |
Intravenously at 3-92 ug/kg once every three weeks (Q3W) or 30 or 37.50 ug/kg every week (QW).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02588092 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1, open-label, dose-escalation, multicenter study to evaluate the tolerability, safety, pharmacokinetics, and activity of ADCT 301 in patients with relapsed or refractory CD25-positive acute myeloid leukemia (AML) or CD25-positive acute lymphoblastic leukemia.
|
||||
| Primary Endpoint |
Two patients achieved complete responses with incomplete hematologic recovery; one each at 30.00 and 37.50 ug/kg QW.
|
||||
| Other Endpoint |
Of 16 patients with post-baseline disease assessments, two patients treated on the QW dosing regimen had a complete response (CR).
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [14] | ||||
| Patients Enrolled |
Relapsed or refractory classical Hodgkin lymphoma and non-Hodgkin lymphoma.
|
||||
| Administration Dosage |
45 ug/kg Q3W for 2 cycles and then 30 ug/kg Q3W thereafter; intravenous administration.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02432235 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1 adaptive dose-escalation study to evaluate the tolerability, safety, pharmacokinetics, and antitumor activity of ADCT-301 in patients with relapsed or refractory Hodgkin lymphoma and non-Hodgkin lymphoma.
|
||||
| Primary Endpoint |
The ORR was 40%,representing 51 responders (patients achieved a best overall response of confirmed PR).
|
||||
| Other Endpoint |
Of the 130 patients included in the safety analysis, 27 (20.77%) experienced grade2 increased GGT, 17 (13.08%) experienced a grade2 neurologic AE,and 18 (13.85%) experienced a grade2 autoimmune AE at cycle 6.
|
||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 11.60% (Day 21) | Moderate CD25 expression (CD25++; 76,000 CD25 molecules/cell) | ||
| Method Description |
Camidanlumab tesirine induces efficient tumor cell killing in CDX models of an anaplastic large cell lymphoma (ALCL) cell line Karpas 299 with moderate CD25 expression (molecules per cell surface=76000). Compared with injection of vehicle (PBS), ADCT-301 administered intravenously (i.v.) at a mean tumor volume of 160 mm3 as a single dose at 0.1 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Anaplastic large cell lymphoma CDX model | ||||
| In Vitro Model | ALK-positive anaplastic large cell lymphoma | Karpas-299 cells | CVCL_1324 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 41.50% (Day 32) | High CD25 expression (CD25+++; 310,000 CD25 molecules/cell) | ||
| Method Description |
Camidanlumab tesirine induces efficient tumor cell killing in CDX models of an anaplastic large cell lymphoma (ALCL) cell line Su-DHL-1 with high CD25 expression (molecules per cell surface=310000).ADCT-301 was administered intravenously at mean tumor volume of 155 mm3 at single doses of 0.3 mg/kg and tumor growth compared with that observed after injection of vehicle (PBS).
Click to Show/Hide
|
||||
| In Vivo Model | Anaplastic large cell lymphoma CDX model | ||||
| In Vitro Model | Anaplastic large cell lymphoma | SU-DHL-1 cells | CVCL_0538 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 51.30% (Day 21) | High CD25 expression (CD25+++; 310,000 CD25 molecules/cell) | ||
| Method Description |
Camidanlumab tesirine induces efficient tumor cell killing in CDX models of an anaplastic large cell lymphoma (ALCL) cell line Su-DHL-1 with high CD25 expression (molecules per cell surface=310000).ADCT-301 was administered intravenously at mean tumor volume of 155 mm3 at single doses of 0.3 mg/kg and tumor growth compared with that observed after injection of vehicle (PBS).
Click to Show/Hide
|
||||
| In Vivo Model | Anaplastic large cell lymphoma CDX model | ||||
| In Vitro Model | Anaplastic large cell lymphoma | SU-DHL-1 cells | CVCL_0538 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 68.40% (Day 21) | Moderate CD25 expression (CD25++; 76,000 CD25 molecules/cell) | ||
| Method Description |
Camidanlumab tesirine induces efficient tumor cell killing in CDX models of an anaplastic large cell lymphoma (ALCL) cell line Karpas 299 with moderate CD25 expression (molecules per cell surface=76000). Compared with injection of vehicle (PBS), ADCT-301 administered intravenously (i.v.) at a mean tumor volume of 160 mm3 as a single dose at 0.2 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Anaplastic large cell lymphoma CDX model | ||||
| In Vitro Model | ALK-positive anaplastic large cell lymphoma | Karpas-299 cells | CVCL_1324 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.20% (Day 21) | Moderate CD25 expression (CD25++; 76,000 CD25 molecules/cell) | ||
| Method Description |
Camidanlumab tesirine induces efficient tumor cell killing in CDX models of an anaplastic large cell lymphoma (ALCL) cell line Karpas 299 with moderate CD25 expression (molecules per cell surface=76000). Compared with injection of vehicle (PBS), ADCT-301 administered intravenously (i.v.) at a mean tumor volume of 160 mm3 as a single dose at 0.4 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Anaplastic large cell lymphoma CDX model | ||||
| In Vitro Model | ALK-positive anaplastic large cell lymphoma | Karpas-299 cells | CVCL_1324 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.10% (Day 32) | High CD25 expression (CD25+++; 310,000 CD25 molecules/cell) | ||
| Method Description |
Camidanlumab tesirine induces efficient tumor cell killing in CDX models of an anaplastic large cell lymphoma (ALCL) cell line Su-DHL-1 with high CD25 expression (molecules per cell surface=310000).ADCT-301 was administered intravenously at mean tumor volume of 155 mm3 at single doses of 0.6 mg/kg and tumor growth compared with that observed after injection of vehicle (PBS).
Click to Show/Hide
|
||||
| In Vivo Model | Anaplastic large cell lymphoma CDX model | ||||
| In Vitro Model | Anaplastic large cell lymphoma | SU-DHL-1 cells | CVCL_0538 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.10% (Day 21) | Moderate CD25 expression (CD25++; 76,000 CD25 molecules/cell) | ||
| Method Description |
Camidanlumab tesirine induces efficient tumor cell killing in CDX models of an anaplastic large cell lymphoma (ALCL) cell line Karpas 299 with moderate CD25 expression (molecules per cell surface=76000).Vechicle (PBS), ADCT-301 (dar 2.2), nonbinding ADC (DAR 2.1) or Adcetris (DAR~4) were administered intravenously at a mean Karpas 299 tumor volume of 130mm3 as single doses at 0.5 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Anaplastic large cell lymphoma CDX model | ||||
| In Vitro Model | ALK-positive anaplastic large cell lymphoma | Karpas-299 cells | CVCL_1324 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.40% (Day 21) | Moderate CD25 expression (CD25++; 76,000 CD25 molecules/cell) | ||
| Method Description |
Camidanlumab tesirine induces efficient tumor cell killing in CDX models of an anaplastic large cell lymphoma (ALCL) cell line Karpas 299 with moderate CD25 expression (molecules per cell surface=76000). Compared with injection of vehicle (PBS), ADCT-301 administered intravenously (i.v.) at a mean tumor volume of 160 mm3 as a single dose at 0.6mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Anaplastic large cell lymphoma CDX model | ||||
| In Vitro Model | ALK-positive anaplastic large cell lymphoma | Karpas-299 cells | CVCL_1324 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.80% (Day 21) | High CD25 expression (CD25+++; 310,000 CD25 molecules/cell) | ||
| Method Description |
Camidanlumab tesirine induces efficient tumor cell killing in CDX models of an anaplastic large cell lymphoma (ALCL) cell line Su-DHL-1 with high CD25 expression (molecules per cell surface=310000).ADCT-301 was administered intravenously at mean tumor volume of 155 mm3 at single doses of 0.6 mg/kg and tumor growth compared with that observed after injection of vehicle (PBS).
Click to Show/Hide
|
||||
| In Vivo Model | Anaplastic large cell lymphoma CDX model | ||||
| In Vitro Model | Anaplastic large cell lymphoma | SU-DHL-1 cells | CVCL_0538 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.26 pM
|
Moderate CD25 expression (CD25++; 17,000 CD25 molecules/cell) | ||
| Method Description |
The inhibitory activity of ADCT-301 against cancer cell growth was evaluated in various human cancer cell lines in vitro. CD25-positive and -negative cell lines were incubated with increasing concentrations of ADCT-301 or free warhead (SG3199) for 96 hours before processing by the MTS assay.
|
||||
| In Vitro Model | Chronic eosinophilic leukemia | EoL-1 cells | CVCL_0258 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
4.96 pM
|
High CD25 expression (CD25+++; 310,000 CD25 molecules/cell) | ||
| Method Description |
The inhibitory activity of ADCT-301 against cancer cell growth was evaluated in various human cancer cell lines in vitro. CD25-positive and -negative cell lines were incubated with increasing concentrations of ADCT-301 or free warhead (SG3199) for 96 hours before processing by the MTS assay.
|
||||
| In Vitro Model | Anaplastic large cell lymphoma | SU-DHL-1 cells | CVCL_0538 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
17.07 pM
|
Moderate CD25 expression (CD25++; 76,000 CD25 molecules/cell) | ||
| Method Description |
The inhibitory activity of ADCT-301 against cancer cell growth was evaluated in various human cancer cell lines in vitro. CD25-positive and -negative cell lines were incubated with increasing concentrations of ADCT-301 or free warhead (SG3199) for 96 hours before processing by the MTS assay.
|
||||
| In Vitro Model | ALK-positive anaplastic large cell lymphoma | Karpas-299 cells | CVCL_1324 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
19.60 pM
|
High CD25 expression (CD25+++; 167,000 CD25 molecules/cell) | ||
| Method Description |
The inhibitory activity of ADCT-301 against cancer cell growth was evaluated in various human cancer cell lines in vitro. CD25-positive and -negative cell lines were incubated with increasing concentrations of ADCT-301 or free warhead (SG3199) for 96 hours before processing by the MTS assay.
|
||||
| In Vitro Model | Hodgkin lymphoma | HDLM-2 cells | CVCL_0009 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) | > 6667.00 pM | Negative CD25 expression (CD25-; <1,000 CD25 molecules/cell) | ||
| Method Description |
The inhibitory activity of ADCT-301 against cancer cell growth was evaluated in various human cancer cell lines in vitro. CD25-positive and -negative cell lines were incubated with increasing concentrations of ADCT-301 or free warhead (SG3199) for 96 hours before processing by the MTS assay.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) | > 6667.00 pM | Negative CD25 expression (CD25-; <1,000 CD25 molecules/cell) | ||
| Method Description |
The inhibitory activity of ADCT-301 against cancer cell growth was evaluated in various human cancer cell lines in vitro. CD25-positive and -negative cell lines were incubated with increasing concentrations of ADCT-301 or free warhead (SG3199) for 96 hours before processing by the MTS assay.
|
||||
| In Vitro Model | Burkitt lymphoma | Daudi cells | CVCL_0008 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) | > 6667.00 pM | Negative CD25 expression (CD25-; <1,000 CD25 molecules/cell) | ||
| Method Description |
The inhibitory activity of ADCT-301 against cancer cell growth was evaluated in various human cancer cell lines in vitro. CD25-positive and -negative cell lines were incubated with increasing concentrations of ADCT-301 or free warhead (SG3199) for 96 hours before processing by the MTS assay.
|
||||
| In Vitro Model | T lymphocytic leukemia | HuT 78 cells | CVCL_0337 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) | > 6667.00 pM | Negative CD25 expression (CD25-; <1,000 CD25 molecules/cell) | ||
| Method Description |
The inhibitory activity of ADCT-301 against cancer cell growth was evaluated in various human cancer cell lines in vitro. CD25-positive and -negative cell lines were incubated with increasing concentrations of ADCT-301 or free warhead (SG3199) for 96 hours before processing by the MTS assay.
|
||||
| In Vitro Model | Adult acute myeloid leukemia | KG-1 cells | CVCL_0374 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
7.49 pM
1.11 ng/mL |
Moderate CD25 expression (CD25++; 96,000 CD25 molecules/cell) | ||
| Method Description |
The inhibitory activity of ADCT-301 against cancer cell growth was evaluated in various human cancer cell lines in vitro. CD25-positive and -negative cell lines were incubated with increasing concentrations of ADCT-301 or free warhead (SG3199) for 96 hours before processing by the MTS assay.
|
||||
| In Vitro Model | Hodgkin lymphoma | L-540 cells | CVCL_1362 | ||
ADCT-602 [Phase 1/2]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Patients Enrolled |
R/R B-acute lymphocytic leukemia (ALL).
|
||||
| Administration Dosage |
A 3+3 dose-escalation design was used for phase 1. ADCT-602 was initially given IV once every 3 weeks (30 ug/kg, n=3; 60 ug/kg, n=4; 90 ug/kg, n=4); based on the PK data, the administration schedule was later amended to weekly infusions (30 ug/kg, n=3; 40 ug/kg, n=4; 50 ug/kg, n=3).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03698552 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1/2 study to evaluate the safety and anti-tumor activity of ADCT-602 targeting CD22 in patients with relapsed or refractory B-cell acute lymphoblastic leukemia.
|
||||
| Primary Endpoint |
In this phase 1 study in pts with very heavily pretreated R/R B-ALL with a median of 5 prior lines of therapy and high baseline bone marrow tumor burden, single-agent ADCT-602 was well tolerated with one pt with DLT noted at the 50 mg/kg weekly dose level. Notably, all 3 pts treated at this dose level had evidence of clinical activity with 2/3 pts achieving MRD negative CR.
Click to Show/Hide
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [17] | ||||
| Patients Enrolled |
R/R B-acute lymphocytic leukemia (ALL).
|
||||
| Administration Dosage |
A 3+3 dose-escalation design was used for phase 1. ADCT-602 was initially given IV once every 3 weeks (30 ug/kg, n=3; 60 ug/kg, n=4; 90 ug/kg, n=4); based on the PK data, the administration schedule was later amended to weekly infusions (30 ug/kg, n=3; 40 ug/kg, n=4; 50 ug/kg, n=3).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03698552 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1/2 study to evaluate the safety and anti-tumor activity of ADCT-602 targeting CD22 in patients with relapsed or refractory b-cell acute lymphoblastic leukemia.
|
||||
| Primary Endpoint |
In this phase 1 study in pts with very heavily pretreated R/R B-ALL with a median of 5 prior lines of therapy and high baseline bone marrow tumor burden, single-agent ADCT-602 was well tolerated with no DLTs noted. Two pts achieved MRD-negative remission. Dose escalation in the weekly schedule continues and 2 additional dose levels (40 ug/kg weekly and 50 ug/kg weekly) are planned. PK data, available for 9 pts treated at every 3-week schedule [30 ug/kg, n=3; 60 ug/kg, n=4; 90 ug/kg, n=2] showed rapid clearance of antibody with mean apparent half-life of <1 day during Cycle 1. This supported transitioning ADCT-602 administration to the weekly dosing.
Click to Show/Hide
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [18] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03698552 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1/2 study to evaluate the safety and anti-tumor activity of ADCT-602 targeting CD22 in patients with relapsed or refractory B-cell acute lymphoblastic leukemia.
|
||||
TR1801-ADC [Phase 1]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [19] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03859752 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1, open label, first-in-human study of TR1801-ADC, an antibody drug conjugate (ADC), in patients with select solid tumors expressing c-met.
|
||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 1.02% (Day 25) | High MET expression (MET+++) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.125 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Colorectal cancer PDX model (PDX: CR3150) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 30.20% (Day 28) | High MET expression (MET+++) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.125 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Head and neck squamous cell carcinoma PDX model (PDX: HN0635) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 30.40% (Day 45) | Moderate MET expression (MET++) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.125 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Head and neck squamous cell carcinoma PDX model (PDX: HN0696) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 34.80% (Day 42) | Moderate MET expression (MET++) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.25 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Colorectal cancer PDX model (PDX: CR0126) | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 35.40% (Day 28) | Moderate MET expression (MET++) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.125 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Head and neck squamous cell carcinoma PDX model (PDX: HN3533) | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 37.50% (Day 25) | Moderate MET expression (MET++) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.25 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Colorectal cancer PDX model (PDX: CR3150) | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 44.60% (Day 42) | High MET expression (MET+++) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.5 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Colorectal cancer PDX model (PDX: CR0126) | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.70% (Day 28) | Moderate MET expression (MET++) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.25 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Head and neck squamous cell carcinoma PDX model (PDX: HN3533) | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.70% (Day 45) | Negative MET expression (MET-) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.25 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Head and neck squamous cell carcinoma PDX model (PDX: HN0696) | ||||
| Experiment 10 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 47.30% (Day 28) | High MET expression (MET+++) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.25 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Head and neck squamous cell carcinoma PDX model (PDX: HN0635) | ||||
| Experiment 11 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 53.20% (Day 28) | Moderate MET expression (MET++) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.5 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Head and neck squamous cell carcinoma PDX model (PDX: HN0635) | ||||
| Experiment 12 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 59.10% (Day 28) | High MET expression (MET+++) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.5 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Head and neck squamous cell carcinoma PDX model (PDX: HN3533) | ||||
| Experiment 13 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 60.40% (Day 45) | High MET expression (MET+++) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.5 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Head and neck squamous cell carcinoma PDX model (PDX: HN0696) | ||||
| Experiment 14 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 70.30% (Day 25) | High MET expression (MET+++; IHC H-score=300) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.5 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Colorectal cancer PDX model (PDX: CR3150) | ||||
| Experiment 15 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 70.80% (Day 28) | High MET expression (MET+++; IHC H-score=295) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 1 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Head and neck squamous cell carcinoma PDX model (PDX: HN0635) | ||||
| Experiment 16 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 70.90% (Day 42) | Moderate MET expression (MET++; IHC H-score=173) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 1 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Colorectal cancer PDX model (PDX: CR0126) | ||||
| Experiment 17 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 76.50% (Day 45) | Moderate MET expression (MET++; IHC H-score=130) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 1 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Head and neck squamous cell carcinoma PDX model (PDX: HN0696) | ||||
| Experiment 18 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 79.40% (Day 60) | Moderate MET expression (MET++; IHC H-score=180) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.125 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: GA0152) | ||||
| Experiment 19 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.50% (Day 28) | Moderate MET expression (MET++; IHC H-score=190) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 1 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Head and neck squamous cell carcinoma PDX model (PDX: HN3533) | ||||
| Experiment 20 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.90% (Day 42) | High MET expression (MET+++; IHC H-score=300) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.125 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Colorectal cancer PDX model (PDX: CR0126) | ||||
| Experiment 21 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 81.40% (Day 60) | High MET expression (MET+++; IHC H-score=300) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.125 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: GA3121) | ||||
| Experiment 22 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 89.20% (Day 60) | Moderate MET expression (MET++; IHC H-score=190) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.25 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: GA0152) | ||||
| Experiment 23 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.10% (Day 25) | High MET expression (MET+++; IHC H-score=300) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 1 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Colorectal cancer PDX model (PDX: CR3150) | ||||
| Experiment 24 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.30% (Day 60) | Moderate MET expression (MET++; IHC H-score=180) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.25 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: GA3121) | ||||
| Experiment 25 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.60% (Day 60) | Moderate MET expression (MET++; IHC H-score=130) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.5 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: GA0152) | ||||
| Experiment 26 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.10% (Day 60) | Moderate MET expression (MET++; IHC H-score=130) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 0.5 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: GA3121) | ||||
| Experiment 27 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 60) | Moderate MET expression (MET++) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 1 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: GA3121) | ||||
| Experiment 28 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 60) | Moderate MET expression (MET++) | ||
| Method Description |
Each mouse was subcutaneously inoculated at the right flank with a 23 mm (diameter) tumor piece of one of the tested PDX models. Mice were randomly grouped into six groups (n = 10 animals) according to the tumor size average of 200 mm3.A single dose of test articles was administered intravenously into the tail vein at the dose concentrations indicated. TR1801-ADC 1 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: GA0152) | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
68.30%
|
High MET expression (MET+++; IHC H-score=300) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Pharyngeal squamous cell carcinoma | Detroit 562 cells | CVCL_1171 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
88.80%
|
Moderate MET expression (MET++; IHC H-score=190) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1573 cells | CVCL_1478 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
90.50%
|
High MET expression (MET+++; IHC H-score=295) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
92.40%
|
Moderate MET expression (MET++; IHC H-score=190) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Colon adenocarcinoma | SW480 cells | CVCL_0546 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
92.90%
|
Moderate MET expression (MET++; IHC H-score=180) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Colon adenocarcinoma | SW1417 cells | CVCL_1717 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
95.20%
|
Moderate MET expression (MET++; IHC H-score=173) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung papillary adenocarcinoma | NCI-H441 cells | CVCL_1561 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
95.50%
|
Moderate MET expression (MET++; IHC H-score=180) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1373 cells | CVCL_1465 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
97.40%
|
Moderate MET expression (MET++; IHC H-score=173) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-1 cells | CVCL_0099 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
97.50%
|
High MET expression (MET+++; IHC H-score=300) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-5 cells | CVCL_0078 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
97.60%
|
Moderate MET expression (MET++; IHC H-score=130) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
97.80%
|
High MET expression (MET+++; IHC H-score=295) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Gastric adenocarcinoma | MKN45 cells | CVCL_0434 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
98.10%
|
Moderate MET expression (MET++; IHC H-score=173) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Hypopharyngeal squamous cell carcinoma | FaDu cells | CVCL_1218 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
98.90%
|
High MET expression (MET+++; IHC H-score=295) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Colon carcinoma | HCT 116 cells | CVCL_0291 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
99.10%
|
High MET expression (MET+++; IHC H-score=300) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H747 cells | CVCL_1587 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
99.30%
|
High MET expression (MET+++; IHC H-score=300) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-620 cells | CVCL_5079 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.20 pM
|
Moderate MET expression (MET++; IHC H-score=173) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung papillary adenocarcinoma | NCI-H441 cells | CVCL_1561 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
15.60 pM
|
High MET expression (MET+++; IHC H-score=300) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-5 cells | CVCL_0078 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
15.80 pM
|
High MET expression (MET+++; IHC H-score=295) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Gastric adenocarcinoma | MKN45 cells | CVCL_0434 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
190.20 pM
|
High MET expression (MET+++; IHC H-score=295) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Colon carcinoma | HCT 116 cells | CVCL_0291 | ||
| Experiment 20 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
197.90 pM
|
High MET expression (MET+++; IHC H-score=300) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-620 cells | CVCL_5079 | ||
| Experiment 21 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
327.50 pM
|
Moderate MET expression (MET++; IHC H-score=173) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Hypopharyngeal squamous cell carcinoma | FaDu cells | CVCL_1218 | ||
| Experiment 22 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
346.40 pM
|
Moderate MET expression (MET++; IHC H-score=130) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
| Experiment 23 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1380.00 pM
|
Moderate MET expression (MET++; IHC H-score=190) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Colon adenocarcinoma | SW480 cells | CVCL_0546 | ||
| Experiment 24 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2272.70 pM
|
Moderate MET expression (MET++; IHC H-score=180) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1373 cells | CVCL_1465 | ||
| Experiment 25 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3230.00 pM
|
High MET expression (MET+++; IHC H-score=300) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H747 cells | CVCL_1587 | ||
| Experiment 26 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3494.00 pM
|
Moderate MET expression (MET++; IHC H-score=180) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Colon adenocarcinoma | SW1417 cells | CVCL_1717 | ||
| Experiment 27 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4664.00 pM
|
High MET expression (MET+++; IHC H-score=295) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 28 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
11.03 nM
|
High MET expression (MET+++; IHC H-score=300) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Pharyngeal squamous cell carcinoma | Detroit 562 cells | CVCL_1171 | ||
| Experiment 29 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
13.44 nM
|
Moderate MET expression (MET++; IHC H-score=190) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1573 cells | CVCL_1478 | ||
| Experiment 30 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
24.37 nM
|
Moderate MET expression (MET++; IHC H-score=173) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-1 cells | CVCL_0099 | ||
ADCT-502 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
20.00% (150 ug/kg)
|
|||
| Patients Enrolled |
Patients with HER2-positive advanced solid tumors.
|
||||
| Administration Dosage |
Seven dose cohorts (30, 60, 120, 150, 180, 210, 240 ug/kg on day 1, iv once every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03125200 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1, open-label, dose-escalation study to evaluate the safety, tolerability, pharmacokinetics, and antitumor activity of ADCT-502 in patients with advanced solid tumors with HER2 expression.
|
||||
MEDI-2228 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [22] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
61.00% (MTD)
|
|||
| Patients Enrolled |
Eligible pts were 18 years old with confirmed relapsed/refractory multiple myeloma (R/R MM) as defined by International Myeloma Working Group consensus criteria, had measurable disease, and had an Eastern Cooperative Oncology Group performance status 1. Pts had to have progressed after treatment with three classes of standard-of-care anti-myeloma drugs, including proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), and monoclonal antibodies (mAbs).
Click to Show/Hide
|
||||
| Administration Dosage |
Administered in the dose of 0.0125-0.20 mg/kg intravenously every 3 weeks (Q3W); The maximum tolerated dose was 0.14 mg/kg Q3W.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03489525 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1, open-label study to evaluate the safety, pharmacokinetics, immunogenicity, and preliminary efficacy of MEDI2228 in subjects with relapsed/refractory multiple myeloma.
|
||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [25] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 82.05% (Day 31) | |||
| Method Description |
The inhibitory activity of MEDI-2228 against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.3 mg/kg MEDI-2228.
|
||||
| In Vivo Model | NSG mice model | ||||
| In Vitro Model | Plasma cell myeloma | MM1.S cells | CVCL_8792 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [25] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.46% (Day 31) | |||
| Method Description |
The inhibitory activity of MEDI-2228+NK against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.3 mg/kg MEDI-2228.
|
||||
| In Vivo Model | NSG mice model | ||||
| In Vitro Model | Plasma cell myeloma | MM1.S cells | CVCL_8792 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [25] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.94% (Day 31) | |||
| Method Description |
The inhibitory activity of MEDI-2228+NK+Dara against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.3 mg/kg MEDI-2228.
|
||||
| In Vivo Model | NSG mice model | ||||
| In Vitro Model | Plasma cell myeloma | MM1.S cells | CVCL_8792 | ||
Obtained from the Model Organism Data
| Experiment 1 Reporting the Activity Date of This ADC | [26] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 67.99% (Day 24) | |||
| Method Description |
The inhibitory activity of MEDI-2228 against cancer cell growth was evaluated in various human cancer cell lines in vivo.The cells were treated with 0.4 mg/kg MEDI-2228.
|
||||
| In Vivo Model | CB17 SCID mice model | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [26] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.12% (Day 24) | |||
| Method Description |
The inhibitory activity combination of M2 and btz for 2 weeks against cancer cell growth was evaluated in various human cancer cell lines in vivo.
|
||||
| In Vivo Model | CB17 SCID mice model | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [26] | ||||
| Efficacy Data | Half Maximal Effective Dose (EC50) |
189.70 ng/mL
|
|||
| Method Description |
The inhibitory activity of MEDI-2228 against cancer cell growth was evaluated in various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Plasma cell myeloma | RPMI-8226 cells | CVCL_0014 | ||
MEDI-3726 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [23] | ||||
| Efficacy Data | Composite Response Rate (RR) |
12.10%
|
|||
| Patients Enrolled |
Patients with metastatic castration-resistant prostate cancer after disease progression on abiraterone and/or enzalutamide and taxane-based chemotherapy.
|
||||
| Administration Dosage |
Administered at 0.015-0.30 mg/kg intravenously every 3 weeks until disease progression/unacceptable toxicity; The MTD was not identified; the MAD was 0.30 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02991911 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1/1b multicenter, open-label, dose-escalation and dose-expansion study to evaluate the safety, pharmacokinetics, immunogenicity, and antitumor activity of MEDI3726 in subjects with metastatic castration resistant prostate cancer who have received prior treatment with abiraterone or enzalutamide.
|
||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [24] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 15.86% (Day 26) | High PSMA expression (PSMA+++; 250,494 PSMA molecules/cell) | ||
| Method Description |
The inhibitory activity of MEDI3726 against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.33 mg/kg MEDI3726.
|
||||
| In Vivo Model | PC-3 CDX model | ||||
| In Vitro Model | Prostate carcinoma | PC-3 cells | CVCL_0035 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [24] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 36.17% (Day 19) | Moderate PSMA expression (PSMA++; 43,766 PSMA molecules/cell) | ||
| Method Description |
The inhibitory activity of MEDI3726 against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.11 mg/kg MEDI3726.
|
||||
| In Vivo Model | PC-3 CDX model | ||||
| In Vitro Model | Prostate carcinoma | LNCaP cells | CVCL_0395 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [24] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.68% (Day 26) | High PSMA expression (PSMA+++; 250,494 PSMA molecules/cell) | ||
| Method Description |
The inhibitory activity of MEDI3726 against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 1 mg/kg MEDI3726.
|
||||
| In Vivo Model | PC-3 CDX model | ||||
| In Vitro Model | Prostate carcinoma | PC-3 cells | CVCL_0035 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [24] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 68.20% (Day 19) | Moderate PSMA expression (PSMA++; 43,766 PSMA molecules/cell) | ||
| Method Description |
The inhibitory activity of MEDI3726 against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.33 mg/kg MEDI3726.
|
||||
| In Vivo Model | PC-3 CDX model | ||||
| In Vitro Model | Prostate carcinoma | LNCaP cells | CVCL_0395 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [24] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 76.95% (Day 19) | High PSMA expression (PSMA+++; 250,494 PSMA molecules/cell) | ||
| Method Description |
The inhibitory activity of MEDI3726 against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 1 mg/kg MEDI3726.
|
||||
| In Vivo Model | PC-3 CDX model | ||||
| In Vitro Model | Prostate carcinoma | LNCaP cells | CVCL_0395 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [24] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.55% (Day 20) | Negative PSMA expression (PSMA-) | ||
| Method Description |
The inhibitory activity of MEDI3726 against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.33 mg/kg MEDI3726.
|
||||
| In Vivo Model | PC-3 CDX model | ||||
| In Vitro Model | Prostate carcinoma | 22RV1 cells | CVCL_1045 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [24] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.71% (Day 20) | Negative PSMA expression (PSMA-) | ||
| Method Description |
The inhibitory activity of MEDI3726 against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 1 mg/kg MEDI3726.
|
||||
| In Vivo Model | PC-3 CDX model | ||||
| In Vitro Model | Prostate carcinoma | 22RV1 cells | CVCL_1045 | ||
Trastuzumab-C239I-SG3400 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 40.50% (Day 50) | Negative expression (HER2-) | ||
| Method Description |
The antitumor activity of trastuzumab-C239i-SG3400 was evaluated in the low-HER2-expressing,trastuzumab-resistant human breast cancer model JIMT-1 and the HER2-positive human gastric cancer xenograft model NCI-N87. A sub-curative single dose of ADC of 1 mg/kg was chosen for both ADCs to observe any difference in activity.
|
||||
| In Vivo Model | Breast cancer CDX model | ||||
| In Vitro Model | Breast cancer | Breast cancer cells | Homo sapiens | ||
| Experiment 2 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 48.00% (Day 50) | Low HER2 expression (HER2+) | ||
| Method Description |
The antitumor activity of trastuzumab-C239i-SG3400 was evaluated in the low-HER2-expressing,trastuzumab-resistant human breast cancer model JIMT-1 and the HER2-positive human gastric cancer xenograft model NCI-N87. A sub-curative single dose of ADC of 1 mg/kg was chosen for both ADCs to observe any difference in activity.
|
||||
| In Vivo Model | Gastric cancer CDX model | ||||
| In Vitro Model | Gastric cancer | Gastric cancer cells | Homo sapiens | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
10.70 ng/mL
|
Low HER2 expression (HER2+) | ||
| Method Description |
The cytotoxicity of the HER2-targeting ADCs was examined in vitro. Every cell line after treatment for 144h with doses ranging from 0.01 to 10, 000 ng/mL. Results are meanSEM of three replicate experiments.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
20.90 ng/mL
|
Low HER2 expression (HER2+) | ||
| Method Description |
The cytotoxicity of the HER2-targeting ADCs was examined in vitro. Every cell line after treatment for 144h with doses ranging from 0.01 to 10, 000 ng/mL. Results are meanSEM of three replicate experiments.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
23.30 ng/mL
|
Low HER2 expression (HER2+) | ||
| Method Description |
The cytotoxicity of the HER2-targeting ADCs was examined in vitro. Every cell line after treatment for 144h with doses ranging from 0.01 to 10, 000 ng/mL. Results are meanSEM of three replicate experiments.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 50.00 ng/mL | Negative expression (HER2-) | ||
| Method Description |
The cytotoxicity of the HER2-targeting ADCs was examined in vitro. Every cell line after treatment for 144h with doses ranging from 0.01 to 10, 000 ng/mL. Results are meanSEM of three replicate experiments.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 250.00 ng/mL | Negative expression (HER2-) | ||
| Method Description |
The cytotoxicity of the HER2-targeting ADCs was examined in vitro. Every cell line after treatment for 144h with doses ranging from 0.01 to 10, 000 ng/mL. Results are meanSEM of three replicate experiments.
|
||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
Trastuzumab-C239I-SG3600 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 62.20% (Day 50) | Low HER2 expression (HER2+) | ||
| Method Description |
The antitumor activity of trastuzumab-C239i-SG3600 was evaluated in the low-HER2-expressing,trastuzumab-resistant human breast cancer model JIMT-1 and the HER2-positive human gastric cancer xenograft model NCI-N87. A sub-curative single dose of ADC of 1 mg/kg was chosen for both ADCs to observe any difference in activity.
|
||||
| In Vivo Model | Gastric cancer CDX model | ||||
| In Vitro Model | Gastric cancer | Gastric cancer cells | Homo sapiens | ||
| Experiment 2 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 65.10% (Day 50) | Negative expression (HER2-) | ||
| Method Description |
The antitumor activity of trastuzumab-C239i-SG3600 was evaluated in the low-HER2-expressing,trastuzumab-resistant human breast cancer model JIMT-1 and the HER2-positive human gastric cancer xenograft model NCI-N87. A sub-curative single dose of ADC of 1 mg/kg was chosen for both ADCs to observe any difference in activity.
|
||||
| In Vivo Model | Breast cancer CDX model | ||||
| In Vitro Model | Breast cancer | Breast cancer cells | Homo sapiens | ||
| Experiment 3 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 50) | Low HER2 expression (HER2+) | ||
| Method Description |
The antitumor activity of trastuzumab-C239i-SG3600 was evaluated in the low-HER2-expressing,trastuzumab-resistant human breast cancer model JIMT-1 and the HER2-positive human gastric cancer xenograft model NCI-N87. A sub-curative single dose of ADC of 3 mg/kg was chosen for both ADCs to observe any difference in activity.
|
||||
| In Vivo Model | Gastric cancer CDX model | ||||
| In Vitro Model | Gastric cancer | Gastric cancer cells | Homo sapiens | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
16.10 ng/mL
|
Low HER2 expression (HER2+) | ||
| Method Description |
The cytotoxicity of the HER2-targeting ADCs was examined in vitro. Every cell line after treatment for 144h with doses ranging from 0.01 to 10, 000 ng/mL. Results are meanSEM of three replicate experiments.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
24.10 ng/mL
|
Low HER2 expression (HER2+) | ||
| Method Description |
The cytotoxicity of the HER2-targeting ADCs was examined in vitro. Every cell line after treatment for 144h with doses ranging from 0.01 to 10, 000 ng/mL. Results are meanSEM of three replicate experiments.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
33.90 ng/mL
|
Low HER2 expression (HER2+) | ||
| Method Description |
The cytotoxicity of the HER2-targeting ADCs was examined in vitro. Every cell line after treatment for 144h with doses ranging from 0.01 to 10, 000 ng/mL. Results are meanSEM of three replicate experiments.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 ug/mL | Negative expression (HER2-) | ||
| Method Description |
The cytotoxicity of the HER2-targeting ADCs was examined in vitro. Every cell line after treatment for 144h with doses ranging from 0.01 to 10, 000 ng/mL. Results are meanSEM of three replicate experiments.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 ug/mL | Negative expression (HER2-) | ||
| Method Description |
The cytotoxicity of the HER2-targeting ADCs was examined in vitro. Every cell line after treatment for 144h with doses ranging from 0.01 to 10, 000 ng/mL. Results are meanSEM of three replicate experiments.
|
||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
Trastuzumab-Flexmab-SG3710 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [28] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 82.20% (Day 50) | Low HER2 expression (HER2+) | ||
| Method Description |
The in vivo efficacy of trastuzumab-Flexmab-SG3710 was investigated after single-dose injections (0.30 mg/kg) in female athymic mice bearing NCI-N87 HER2-positive subcutaneous xenografts. NCI-N87 cells (5 x106) in 50% Matrigel were inoculated subcutaneously into 46 week-old female athymic nude mice. Five mice per group were dosed intravenously five days after their tumors reached a volume of 200 mm3.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [28] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 92.20% (Day 50) | Low HER2 expression (HER2+) | ||
| Method Description |
The in vivo efficacy of trastuzumab-Flexmab-SG3710 was investigated after single-dose injections (0.6 mg/kg) in female athymic mice bearing NCI-N87 HER2-positive subcutaneous xenografts. NCI-N87 cells (5 x106) in 50% Matrigel were inoculated subcutaneously into 46 week-old female athymic nude mice. Five mice per group were dosed intravenously five days after their tumors reached a volume of 200 mm3.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [28] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 92.80% (Day 40) | Low HER2 expression (HER2+) | ||
| Method Description |
The in vivo efficacy of trastuzumab-Flexmab-SG3710 and trastuzumab-C239i-SG3249 ADCs was investigated after single-dose injections (1 mg/kg) in female athymic mice bearing NCI-N87 HER2-positive subcutaneous xenografts,and tumor growth was monitored for 85 days. NCI-N87 cells (5 x106) in 50% Matrigel were inoculated subcutaneously into 46 week-old female athymic nude mice. Five mice per group were dosed intravenously five days after their tumors reached a volume of 200 mm3.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [28] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
35.50 pM
|
Low HER2 expression (HER2+) | ||
| Method Description |
The cytotoxic effect of Trastuzumab-Flexmab-SG3710 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing breast and gastric cancers. The potency of trastuzumab-Flexmab-SG3710 was assessed on the SKBR-3 cell line after five days of incubation.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
MEDI-0641 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [29] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 91.30% (Day 49) | Positive 5T4 expression (5T4+++/++) | ||
| Method Description |
A single dose of 5T4-PBD was administrated at 0.1 and 0.3 mg/kg intravenously into nude mice.
|
||||
| In Vivo Model | MDA-MB-361 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-361 cells | CVCL_0620 | ||
Trastuzumab-C239I-SG3249 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [28] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.00% (Day 50) | Low HER2 expression (HER2+) | ||
| Method Description |
The in vivo efficacy of trastuzumab-C239i-SG3249 was investigated after single-dose injections (0.30 mg/kg) in female athymic mice bearing NCI-N87 HER2-positive subcutaneous xenografts. NCI-N87 cells (5 x106) in 50% Matrigel were inoculated subcutaneously into 46 week-old female athymic nude mice. Five mice per group were dosed intravenously five days after their tumors reached a volume of 200 mm3.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [28] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.20% (Day 40) | Low HER2 expression (HER2+) | ||
| Method Description |
The in vivo efficacy of trastuzumab-Flexmab-SG3710 and trastuzumab-C239i-SG3249 ADCs was investigated after single-dose injections (1 mg/kg) in female athymic mice bearing NCI-N87 HER2-positive subcutaneous xenografts,and tumor growth was monitored for 85 days. NCI-N87 cells (5 x106) in 50% Matrigel were inoculated subcutaneously into 46 week-old female athymic nude mice. Five mice per group were dosed intravenously five days after their tumors reached a volume of 200 mm3.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [28] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
14.40 pM
|
Low HER2 expression (HER2+) | ||
| Method Description |
The cytotoxic effect of Trastuzumab-C239i-SG32490 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing breast and gastric cancers. The potency of trastuzumab-Flexmab-SG3710 was assessed on the SKBR-3 cell line after five days of incubation.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
chDAB4-SG3249 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [30] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.50% (Day 14) | Positive La/SSB expression (La/SSB +++/++) | ||
| Method Description |
ChDAB4-SG3249 (3 mg/kg, the day after chemotherapy (Day 3)) induces efficient tumor cell killing in cell line-derived models of Lewis lung carcinoma cell with HER3 expression with high expression.
|
||||
| In Vivo Model | LL2 CDX model | ||||
| In Vitro Model | Normal | LL2 cells | CVCL_C4MM | ||
HD12-SG3315 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
41.50 pM
|
Moderate MET expression (MET++; IHC H-score=180) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1373 cells | CVCL_1465 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
46.00 pM
|
Moderate MET expression (MET++; IHC H-score=130) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
19.70 pM
|
Moderate MET expression (MET++; IHC H-score=180) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1373 cells | CVCL_1465 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.15 nM
|
Moderate MET expression (MET++; IHC H-score=130) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
HD12-SG3246 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
49.00 pM
|
Moderate MET expression (MET++; IHC H-score=180) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1373 cells | CVCL_1465 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
61.50 pM
|
Moderate MET expression (MET++; IHC H-score=130) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
11.10 pM
|
Moderate MET expression (MET++; IHC H-score=180) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1373 cells | CVCL_1465 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
91.00 pM
|
Moderate MET expression (MET++; IHC H-score=130) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
HD12-SG3259 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
56.00 pM
|
Moderate MET expression (MET++; IHC H-score=130) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
77.50 pM
|
Moderate MET expression (MET++; IHC H-score=180) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1373 cells | CVCL_1465 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
24.40 pM
|
Moderate MET expression (MET++; IHC H-score=130) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
31.30 pM
|
Moderate MET expression (MET++; IHC H-score=180) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1373 cells | CVCL_1465 | ||
HD12-SG3227 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
66.00 pM
|
Moderate MET expression (MET++; IHC H-score=180) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1373 cells | CVCL_1465 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
76.50 pM
|
Moderate MET expression (MET++; IHC H-score=130) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
27.50 pM
|
Moderate MET expression (MET++; IHC H-score=180) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1373 cells | CVCL_1465 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.41 nM
|
Moderate MET expression (MET++; IHC H-score=130) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
HD12-SG3249 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
76.80 pM
|
Moderate MET expression (MET++; IHC H-score=180) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1373 cells | CVCL_1465 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Maximum inhibition efficiency (MIE) |
78.70 pM
|
Moderate MET expression (MET++; IHC H-score=130) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
21.80 pM
|
Moderate MET expression (MET++; IHC H-score=180) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1373 cells | CVCL_1465 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.15 nM
|
Moderate MET expression (MET++; IHC H-score=130) | ||
| Method Description |
Cell viability was determined by measuring the luminescence after adding the CellTiter-Glo 2.0 reagent. Cancer cells were seeded overnight in growth media and incubated at 37°C, 5% CO2,and 95% humidity. starting with concentrations of 100 nM for ADCs for free drug. Cells were exposed to test articlesfor 5 days.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
HER-SG3249 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [32] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
1.74 ng/mL
|
Low HER2 expression (HER2+) | ||
| Method Description |
The cytotoxic effect of Her-SG3249 was assessed in cell viability assays for breast cancer. The potency of Her-SG3249 was assessed on the SKBR-3 cell line. Process aggregation assessed by SEC.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
Site-specific HER-SG3249 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [32] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
2.62 ng/mL
|
Low HER2 expression (HER2+) | ||
| Method Description |
The cytotoxic effect of site-specific Her-SG3249 was assessed in cell viability assays for breast cancer. The potency of site-specific Her-SG3249 was assessed on the SKBR-3 cell line. Process aggregation assessed by SEC.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
40H3-Tesirine [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.31 nM
|
High EGFR expression (EGFR+++) | ||
| Method Description |
1 x104 cells per well in a volume of 100 ul were plated in 96-well tissue culture plates. After 24 h, ADCs were added at the indicated concentrations. After 72 h, the medium was removed and the viability was determined using the CellTiter-Glo luminescent cell viability assay kit.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.85 nM
|
High EGFR expression (EGFR+++) | ||
| Method Description |
1 x104 cells per well in a volume of 100 ul were plated in 96-well tissue culture plates. After 24 h, ADCs were added at the indicated concentrations. After 72 h, the medium was removed and the viability was determined using the CellTiter-Glo luminescent cell viability assay kit.
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
9.07 nM
|
Moderate EGFR expression (EGFR++) | ||
| Method Description |
1 x104 cells per well in a volume of 100 ul were plated in 96-well tissue culture plates. After 24 h, ADCs were added at the indicated concentrations. After 72 h, the medium was removed and the viability was determined using the CellTiter-Glo luminescent cell viability assay kit.
|
||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-20 cells | CVCL_0178 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100.00 nM | Negative EGFR expression (EGFR-) | ||
| Method Description |
1 x104 cells per well in a volume of 100 ul were plated in 96-well tissue culture plates. After 24 h, ADCs were added at the indicated concentrations. After 72 h, the medium was removed and the viability was determined using the CellTiter-Glo luminescent cell viability assay kit.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
Mil40-8 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [34] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
235.60 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
SKOV-3, BT474 HerDR, MDA-MB-231 and MCF-7 cells were cultured under various concentrations of Mil40, SN-38 and ADCs for 10days, 9days, 6days, and 6days, respectively. Cytotoxicity assays were established using the CellTiter-Go assay kit (CTG).
|
||||
| In Vitro Model | Invasive breast carcinoma | BT474 HerDR cells | CVCL_0179 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [34] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
283.80 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
SKOV-3, BT474 HerDR, MDA-MB-231 and MCF-7 cells were cultured under various concentrations of Mil40, SN-38 and ADCs for 10days, 9days, 6days, and 6days, respectively. Cytotoxicity assays were established using the CellTiter-Go assay kit (CTG).
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [34] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 1000.00 nM | Negative HER2 expression (HER2-) | ||
| Method Description |
SKOV-3, BT474 HerDR, MDA-MB-231 and MCF-7 cells were cultured under various concentrations of Mil40, SN-38 and ADCs for 10days, 9days, 6days, and 6days, respectively. Cytotoxicity assays were established using the CellTiter-Go assay kit (CTG).
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [34] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 1000.00 nM | Negative HER2 expression (HER2-) | ||
| Method Description |
SKOV-3, BT474 HerDR, MDA-MB-231 and MCF-7 cells were cultured under various concentrations of Mil40, SN-38 and ADCs for 10days, 9days, 6days, and 6days, respectively. Cytotoxicity assays were established using the CellTiter-Go assay kit (CTG).
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
Trastuzumab-SG3600 high DAR [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.60 ng/mL
|
Low HER2 expression (HER2+) | ||
| Method Description |
The cytotoxicity of the HER2-targeting ADCs was examined in vitro. Every cell line after treatment for 144h with doses ranging from 0.01 to 10, 000 ng/mL. Results are meanSEM of three replicate experiments.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.37 ng/mL
|
Low HER2 expression (HER2+) | ||
| Method Description |
The cytotoxicity of the HER2-targeting ADCs was examined in vitro. Every cell line after treatment for 144h with doses ranging from 0.01 to 10, 000 ng/mL. Results are meanSEM of three replicate experiments.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.90 ng/mL
|
Low HER2 expression (HER2+) | ||
| Method Description |
The cytotoxicity of the HER2-targeting ADCs was examined in vitro. Every cell line after treatment for 144h with doses ranging from 0.01 to 10, 000 ng/mL. Results are meanSEM of three replicate experiments.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
36.10 ng/mL
|
Negative expression (HER2-) | ||
| Method Description |
The cytotoxicity of the HER2-targeting ADCs was examined in vitro. Every cell line after treatment for 144h with doses ranging from 0.01 to 10, 000 ng/mL. Results are meanSEM of three replicate experiments.
|
||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [27] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 ug/mL | Negative expression (HER2-) | ||
| Method Description |
The cytotoxicity of the HER2-targeting ADCs was examined in vitro. Every cell line after treatment for 144h with doses ranging from 0.01 to 10, 000 ng/mL. Results are meanSEM of three replicate experiments.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
References
