Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0LNARP
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
MEDI-0641
|
|||||
| Synonyms |
MEDI0641; MEDI 0641; 5T4-PBD
Click to Show/Hide
|
|||||
| Organization |
MedImmune LLC; AstraZeneca PLC
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
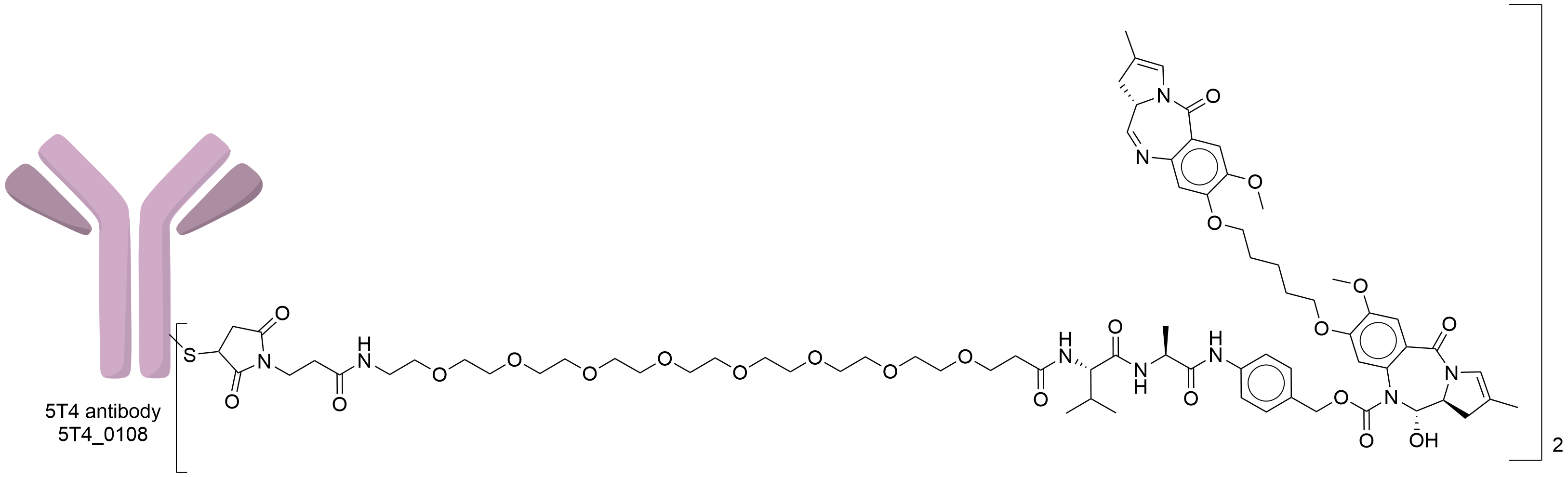
|
|||||
| Antibody Name |
5T4_0108
|
Antibody Info | ||||
| Antigen Name |
Trophoblast glycoprotein (TPBG)
|
Antigen Info | ||||
| Payload Name |
5T4-PBD
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
Mal-PEG8-Val-Ala-PABC
|
Linker Info | ||||
General Information of The Activity Data Related to This ADC
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 91.30% (Day 49) | Positive 5T4 expression (5T4+++/++) | ||
| Method Description |
A single dose of 5T4-PBD was administrated at 0.1 and 0.3 mg/kg intravenously into nude mice.
|
||||
| In Vivo Model | MDA-MB-361 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-361 cells | CVCL_0620 | ||
References
