Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0OHTUI
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Trastuzumab-C239I-SG3249
|
|||||
| Synonyms |
Trastuzumab-C239i-SG3249
Click to Show/Hide
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
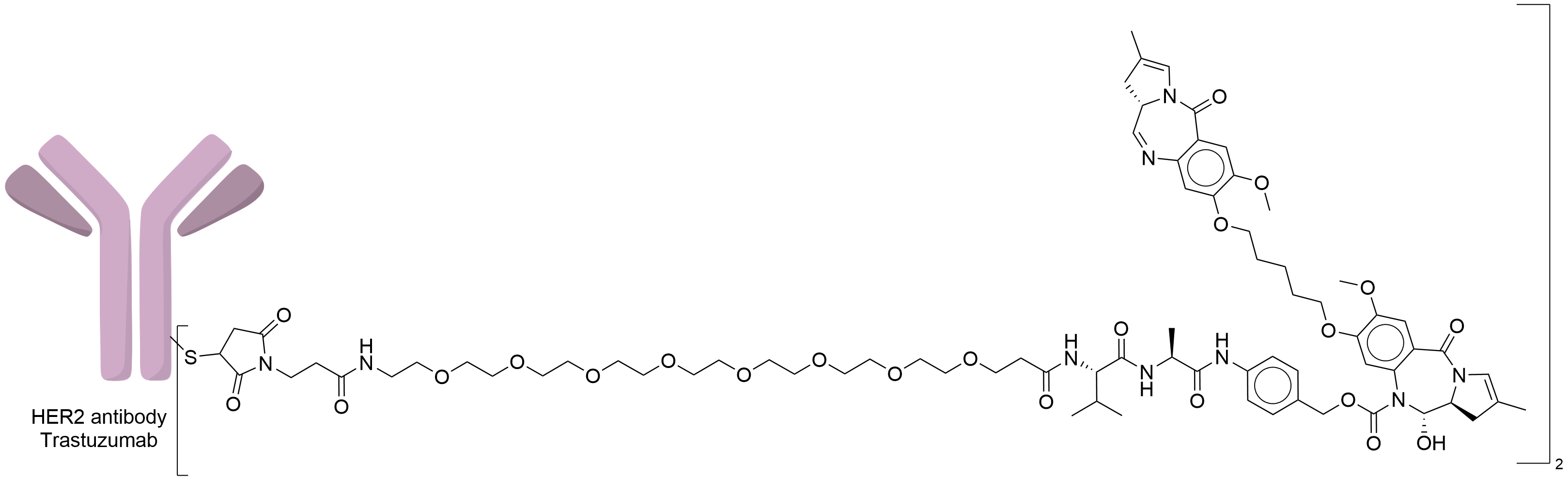
|
|||||
| Antibody Name |
Trastuzumab
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
SG3199
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
Mal-PEG8-Val-Ala-PABC
|
Linker Info | ||||
| Conjugate Type |
Conjudating to SG3249 using sulfhydryl groups obtained by reduction of interchain cysteines in the flexmab hinge region, Michael addition reaction.
|
|||||
| Combination Type |
SG3249
|
|||||
General Information of The Activity Data Related to This ADC
Discovered Using Cell Line-derived Xenograft Model
Revealed Based on the Cell Line Data
| Standard Type | Value | Units | Cell Line | Disease Model |
|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) |
14.4
|
pM
|
SK-BR-3 cells
|
Breast adenocarcinoma
|
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.00% (Day 50) | Low HER2 expression (HER2+) | ||
| Method Description |
The in vivo efficacy of trastuzumab-C239i-SG3249 was investigated after single-dose injections (0.30 mg/kg) in female athymic mice bearing NCI-N87 HER2-positive subcutaneous xenografts. NCI-N87 cells (5 x106) in 50% Matrigel were inoculated subcutaneously into 46 week-old female athymic nude mice. Five mice per group were dosed intravenously five days after their tumors reached a volume of 200 mm3.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.20% (Day 40) | Low HER2 expression (HER2+) | ||
| Method Description |
The in vivo efficacy of trastuzumab-Flexmab-SG3710 and trastuzumab-C239i-SG3249 ADCs was investigated after single-dose injections (1 mg/kg) in female athymic mice bearing NCI-N87 HER2-positive subcutaneous xenografts,and tumor growth was monitored for 85 days. NCI-N87 cells (5 x106) in 50% Matrigel were inoculated subcutaneously into 46 week-old female athymic nude mice. Five mice per group were dosed intravenously five days after their tumors reached a volume of 200 mm3.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 14.40 pM | Low HER2 expression (HER2+) | ||
| Method Description |
The cytotoxic effect of Trastuzumab-C239i-SG32490 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing breast and gastric cancers. The potency of trastuzumab-Flexmab-SG3710 was assessed on the SKBR-3 cell line after five days of incubation.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
References
