Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0NTFBR
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
MEDI-3726
|
|||||
| Synonyms |
ADCT 401; ADCT-401; MEDI 3726; MEDI-3726; MEDI3 726 (forme rly ADCT-401); MEDI3726
Click to Show/Hide
|
|||||
| Organization |
MedImmune LLC; AstraZeneca PLC
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
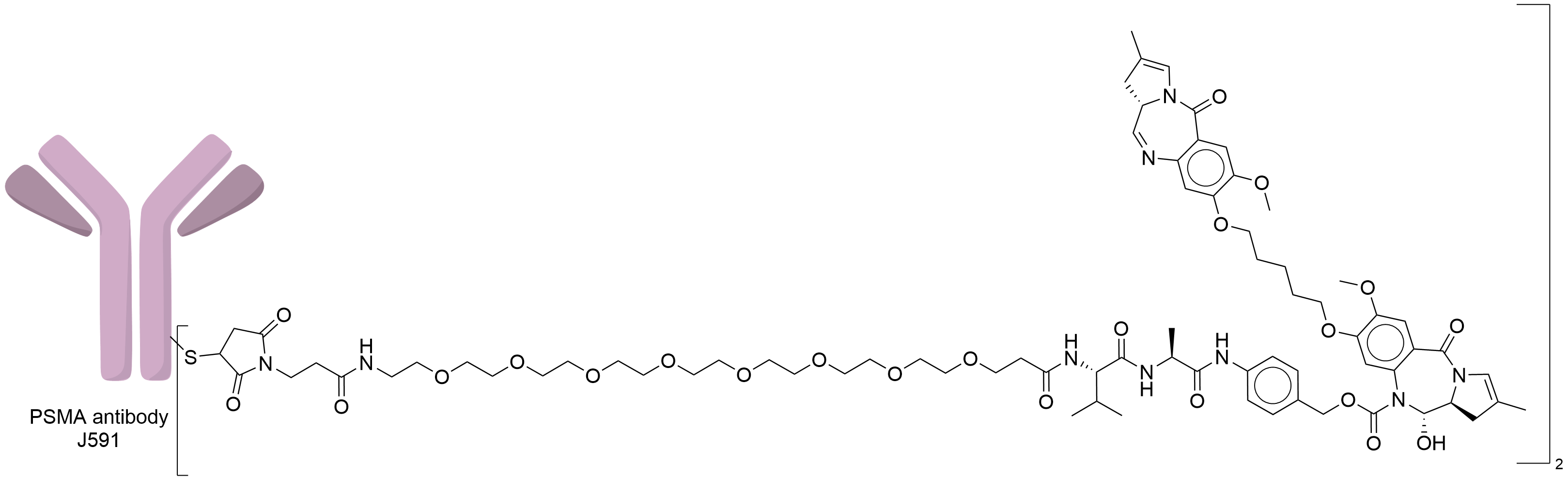
|
|||||
| Antibody Name |
J591
|
Antibody Info | ||||
| Antigen Name |
Glutamate carboxypeptidase 2 (FOLH1)
|
Antigen Info | ||||
| Payload Name |
SG3199
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
Mal-PEG8-Val-Ala-PABC
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
tesirine
|
|||||
| Puchem SID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Cell Line-derived Xenograft Model
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Composite Response Rate (RR) |
12.10%
|
|||
| Patients Enrolled |
Patients with metastatic castration-resistant prostate cancer after disease progression on abiraterone and/or enzalutamide and taxane-based chemotherapy.
|
||||
| Administration Dosage |
Administered at 0.015-0.30 mg/kg intravenously every 3 weeks until disease progression/unacceptable toxicity; The MTD was not identified; the MAD was 0.30 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02991911 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/1b multicenter, open-label, dose-escalation and dose-expansion study to evaluate the safety, pharmacokinetics, immunogenicity, and antitumor activity of MEDI3726 in subjects with metastatic castration resistant prostate cancer who have received prior treatment with abiraterone or enzalutamide. | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 15.86% (Day 26) | High PSMA expression (PSMA+++; 250,494 PSMA molecules/cell) | ||
| Method Description |
The inhibitory activity of MEDI3726 against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.33 mg/kg MEDI3726.
|
||||
| In Vivo Model | PC-3 CDX model | ||||
| In Vitro Model | Prostate carcinoma | PC-3 cells | CVCL_0035 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 36.17% (Day 19) | Moderate PSMA expression (PSMA++; 43,766 PSMA molecules/cell) | ||
| Method Description |
The inhibitory activity of MEDI3726 against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.11 mg/kg MEDI3726.
|
||||
| In Vivo Model | PC-3 CDX model | ||||
| In Vitro Model | Prostate carcinoma | LNCaP cells | CVCL_0395 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.68% (Day 26) | High PSMA expression (PSMA+++; 250,494 PSMA molecules/cell) | ||
| Method Description |
The inhibitory activity of MEDI3726 against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 1 mg/kg MEDI3726.
|
||||
| In Vivo Model | PC-3 CDX model | ||||
| In Vitro Model | Prostate carcinoma | PC-3 cells | CVCL_0035 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 68.20% (Day 19) | Moderate PSMA expression (PSMA++; 43,766 PSMA molecules/cell) | ||
| Method Description |
The inhibitory activity of MEDI3726 against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.33 mg/kg MEDI3726.
|
||||
| In Vivo Model | PC-3 CDX model | ||||
| In Vitro Model | Prostate carcinoma | LNCaP cells | CVCL_0395 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 76.95% (Day 19) | High PSMA expression (PSMA+++; 250,494 PSMA molecules/cell) | ||
| Method Description |
The inhibitory activity of MEDI3726 against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 1 mg/kg MEDI3726.
|
||||
| In Vivo Model | PC-3 CDX model | ||||
| In Vitro Model | Prostate carcinoma | LNCaP cells | CVCL_0395 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.55% (Day 20) | Negative PSMA expression (PSMA-) | ||
| Method Description |
The inhibitory activity of MEDI3726 against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.33 mg/kg MEDI3726.
|
||||
| In Vivo Model | PC-3 CDX model | ||||
| In Vitro Model | Prostate carcinoma | 22RV1 cells | CVCL_1045 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.71% (Day 20) | Negative PSMA expression (PSMA-) | ||
| Method Description |
The inhibitory activity of MEDI3726 against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 1 mg/kg MEDI3726.
|
||||
| In Vivo Model | PC-3 CDX model | ||||
| In Vitro Model | Prostate carcinoma | 22RV1 cells | CVCL_1045 | ||
References
