Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0TKVCB
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Loncastuximab tesirine
|
|||||
| Brand Name |
Zynlonta
|
|||||
| Synonyms |
ADCT 402;ADCT-402;MT-2111;Anti-CD19-PBD-conjugate-ADC;Immunoglobulin g1-kappa;Lonca;Lonca-T;loncastuximab tesirine-lpyl
Click to Show/Hide
|
|||||
| Organization |
ADC Therapeutics SA; BSP Pharmaceuticals SpA; Overland Pharmaceutical (Shanghai) Co., Ltd
|
|||||
| Drug Status |
Approved (FDA):Apr, 2021
|
|||||
| Indication |
In total 5 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2.3
|
|||||
| Structure |
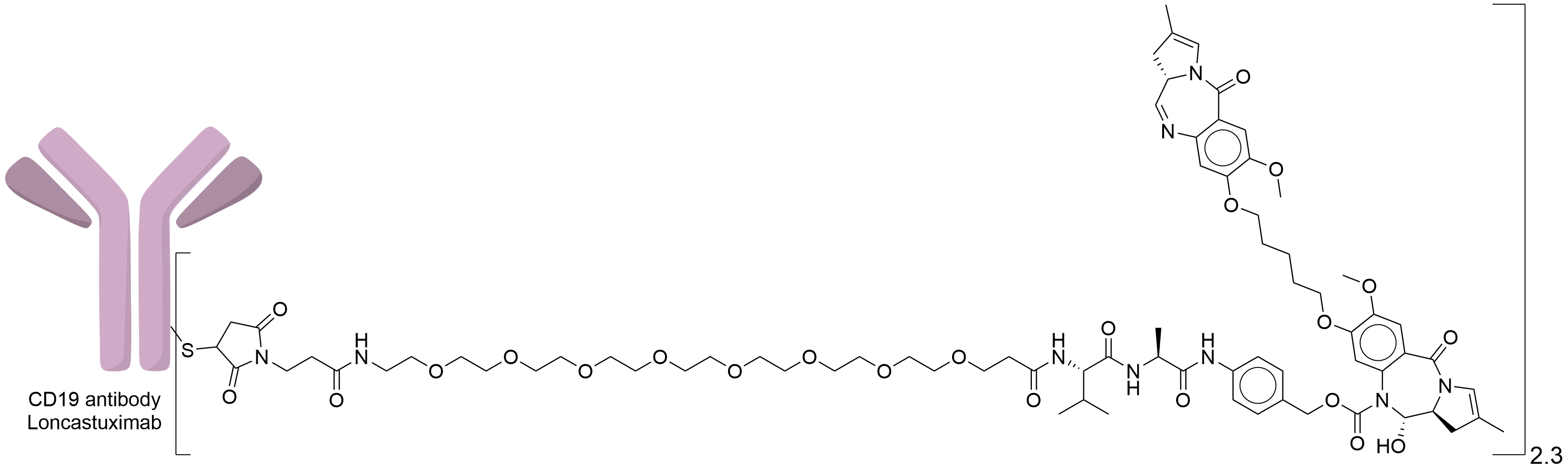
|
|||||
| Antibody Name |
Loncastuximab
|
Antibody Info | ||||
| Antigen Name |
B-lymphocyte antigen CD19 (CD19)
|
Antigen Info | ||||
| Payload Name |
SG3199
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
Mal-PEG8-Val-Ala-PABC
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
Tesirine
|
|||||
| Absorption |
For loncastuximab tesirine, mean Cmax=2995 (ug/L) during cycle 1, mean Cmax=3155 (ug/L) during cycle 2; AUC=15,245-22,823 (day x ug/L).
|
|||||
| Distribution |
The loncastuximab tesirine-lpyl volume of distribution was 7.11 (7.19-8.43) liters.
|
|||||
| Metabolism |
The monoclonal antibody portion of loncastuximab tesirine-lpyl is catabolized into small peptides. In vitro studies show that the small molecule cytotoxin portion, SG3199, is metabolized by CYP3A4/5. The half-life of loncastuximab tesirine-lpyl was 7.06-12.5 days at steady-state.
|
|||||
| Elimination |
The main excretion pathways of SG3199 have not been formally studied in humans. SG3199 is thought to be minimally excreted by the kidneys. The mean clearance of loncastuximab tesirine-lpyl were 0.499 L/day (after a single dose) and 0.275 L/day (at steady-state).
|
|||||
| Toxicity |
LD50 information for loncastuximab tesirine is not readily available in the literature. Toxicity is increased at higher doses, and may lead to discontinuation.
|
|||||
| Special Approval(s) |
Priority review(FDA); Accelerated approval(FDA); Orphan drug(FDA); Orphan drug(EMA)
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| DrugMap ID | ||||||
| TTD ID | ||||||
| DRESIS ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
48.28%
|
|||
| Patients Enrolled |
Relapsed or refractory diffuse large B-cell lymphoma (DLBCL) after two or more multiagent systemic treatments, who had measurable disease and Eastern Cooperative Oncology Group performance status 0-2.
|
||||
| Administration Dosage |
Intravenously on day 1 of each 21-day cycle, at 150 ug/kg for two cycles, then 75 ug/kg thereafter, for up to 1 year or until disease relapse or progression, unacceptable toxicity, death, major protocol deviation, pregnancy, or patient, investigator, or sponsor decision.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03589469 | Clinical Status | Phase 2 | ||
| Clinical Description | A phase 2 open-label single-arm study to evaluate the efficacy and safety of loncastuximab tesirine in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) (LOTIS-2). | ||||
| Primary Endpoint |
Overall response rate assessed by central review. 70 of 145 patients had complete or partial response (overall response rate 48.28% [95% CI 39.9-56.7]); 35 had complete response and 35 had partial response.
|
||||
| Other Endpoint |
Median time to first response (complete response or partial response) was 41.00 days (IQR 38.00-44.00). Median duration of response was 10.30 months (95% CI 6.9-not estimable); 13.40 months (10.30-not estimable) for patients with complete response and 5.70 months (1.70-not estimable) for patients with partial response. The probability of responders maintaining responses for 9 months or longer was 64.0%. Median progressionfree survival was 4.90 months (95% CI 2.90-8.30), median overall survival was 9.90 months (6.70-11.50), and median relapse-free survival was 13.40 months (10.30-not estimable).
Click to Show/Hide
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
59.40%
|
|||
| Patients Enrolled |
R/R B-cell non Hodgkin lymphoma (NHL); had failed or were intolerant to established therapies, or for whom no other established treatment options were available.
|
||||
| Administration Dosage |
5 to 200 ug/kg; intravenously over 1 hour, once every 3 weeks (one cycle) by a 3+3 dose-escalation design.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02669017 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 dose-escalation study to evaluate the tolerability, safety, pharmacokinetics, and antitumor activity of ADCT-402 in patients with relapsed or refractory b-cell lineage non Hodgkin lymphoma (B-NHL). | ||||
| Primary Endpoint |
The MTD was not established during the trial due to the low level of DLTs, 150 ug/kg dose for expansion and phase 2.
|
||||
| Other Endpoint |
For loncastuximab tesirine 120 ug/kg,Median Progression-Free Survival (mPFS)=4.80 months.The median PFS OS=11.60 months.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Complete Remission (CR) |
26.70%
|
|||
| Patients Enrolled |
Adults (age 18 years) with histologically confirmed relapsed/refractory B-non Hodgkin lymphoma (World Health Organization 2008 classification18 ) who were intolerant to established therapy, for whom established therapy had failed, or for whom no other treatment options were available in the opinion of the investigator were eligible to participate.
|
||||
| Administration Dosage |
3 + 3 dose escalation at 15 to 200 ug/kg and dose expansion at 120 and 150 ug/kg; IV infusion over 60 minutes once every 3 weeks (day 1 of each 21-day cycle).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02669017 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 dose-escalation study to evaluate the tolerability, safety, pharmacokinetics, and antitumor activity of ADCT-402 in patients with relapsed or refractory B-cell lineage non Hodgkin lymphoma (B-NHL). | ||||
| Primary Endpoint |
The recommended dose of loncastuximab tesirine for Phase 2 is 150 ug/kg Q3W for 2 doses followed by 75 ug/kg Q3W for subsequent doses. The 150 ug/kg dose was selected as a dose with encouraging responses but lower frequency of AEs than observed with the 200 u/kg dose. Overall response rate (ORR) in evaluable patients was 45.60%, including 26.70% complete responses (CR). Median PFS was 3.10 months (95% CI 2.70-4.20) in all patients with B-NHL, 2.8 months (95% CI 1.90-3.80) in patients with DLBCL, 4.80 months (95% CI 1.10-7.80) in patients with MCL, and could not be determined in those with FL due to the low number of events. Median OS was 8.3 months (95% CI 6.70-10.70) in all patients with B-NHL, 7.5 months (95% CI 6.00-9.80) in patients with DLBCL, and was not reached in patients with MCL or FL due to low number of events.
Click to Show/Hide
|
||||
| Other Endpoint |
PK exposure similarity between loncastuximab tesirine total antibody and PBD-conjugated antibody indicated good stability in serum.Accumulation by Cycle 2 for patients on a Q6W dosing regimen was lower than that of those on Q3W dosing: mean accumulation of 1.22 and 1.33 for PBD-conjugated antibody and total antibody on Q6W regimens compared with 1.72 and 1.74 on Q3W regimens. There was substantial variability in PK exposure and PK parameters assessed for PBD-conjugated antibody, total antibody, and SG3199.
Click to Show/Hide
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Complete Remission (CR) |
40.20%
|
|||
| Patients Enrolled |
R/R B-acute lymphocytic leukemia (ALL) for standard therapies had failed, intolerant to standard therapies, or no other treatment options were available, in the opinion of the investigator, were eligible for the study, other key inclusion criteria included Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2.
|
||||
| Administration Dosage |
15 to 150 ug/kg once every 3 weeks (Q3W) or 50 ug/kg IV weekly.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02669264 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, open-label, adaptive dose-escalation, multicenter study to evaluate the tolerability, safety, pharmacokinetics, and anti-tumor activity of ADCT-402 in patients with relapsed or refractory B-cell lineage acute lymphoblastic leukemia (B-ALL). | ||||
| Primary Endpoint |
Part II (dose expansion portion) utilized the doses of 120 and 150 ug/kg, selected based on antitumor activity and tolerability seen during part I. The overall response rate (ORR) in patients with DLBCL, mantle cell lymphoma, and follicular lymphoma was 42.30%, 46.70%, and 78.60%, respectively.
|
||||
| Other Endpoint |
The median progression-free survival (PFS) was 3.10 months in all patients with B-NHL and 2.80 months in patients with DLBCL, whereas the median OS was 8.30 months in all patients and 7.50 months in patients with DLBCL.
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Complete Remission (CR) |
46.16%
|
|||
References
