Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0IYBRT
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
ADCT-602
|
|||||
| Synonyms |
ADCT-602; Epratuzumab-cys-SG3249; Epratuzumab-cys-tesirine; hLL2-cys-PBD; hLL2-cys-SG3249
Click to Show/Hide
|
|||||
| Organization |
ADC Therapeutics SA
|
|||||
| Drug Status |
Phase 1/2
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
1.7
|
|||||
| Structure |
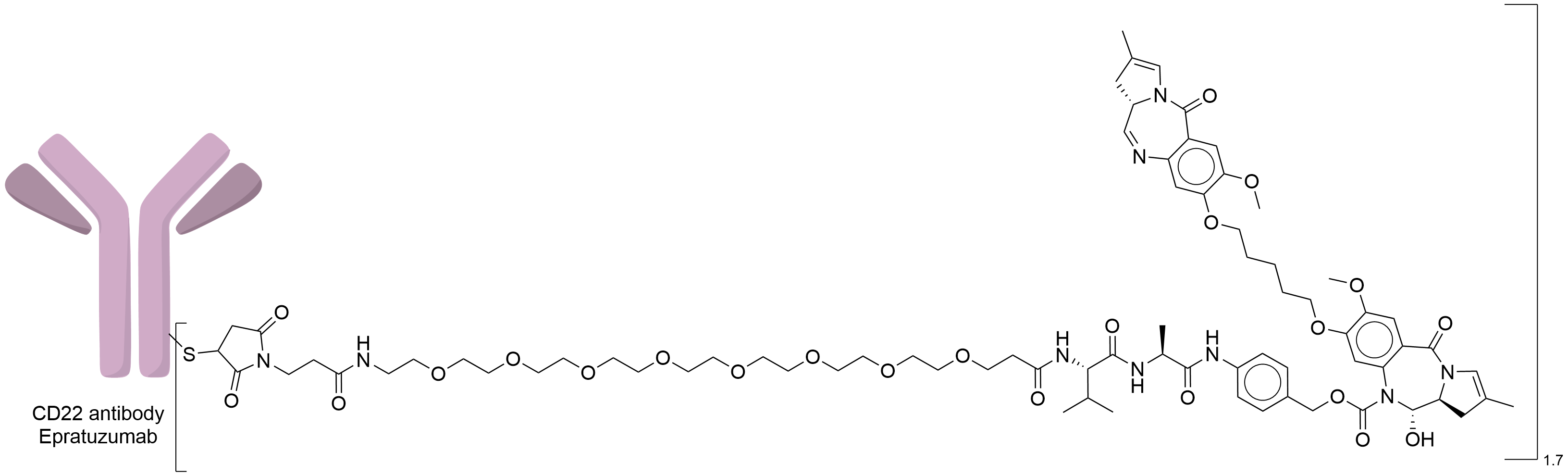
|
|||||
| Antibody Name |
Epratuzumab
|
Antibody Info | ||||
| Antigen Name |
B-cell receptor CD22 (CD22)
|
Antigen Info | ||||
| Payload Name |
SG3199
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
Mal-PEG8-Val-Ala-PABC
|
Linker Info | ||||
| Conjugate Type |
Reactive Cysteines
|
|||||
| Combination Type |
tesirine
|
|||||
| Puchem SID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Patients Enrolled |
R/R B-acute lymphocytic leukemia (ALL).
|
||||
| Administration Dosage |
A 3+3 dose-escalation design was used for phase 1. ADCT-602 was initially given IV once every 3 weeks (30 ug/kg, n=3; 60 ug/kg, n=4; 90 ug/kg, n=4); based on the PK data, the administration schedule was later amended to weekly infusions (30 ug/kg, n=3; 40 ug/kg, n=4; 50 ug/kg, n=3).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03698552 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/2 study to evaluate the safety and anti-tumor activity of ADCT-602 targeting CD22 in patients with relapsed or refractory B-cell acute lymphoblastic leukemia. | ||||
| Primary Endpoint |
In this phase 1 study in pts with very heavily pretreated R/R B-ALL with a median of 5 prior lines of therapy and high baseline bone marrow tumor burden, single-agent ADCT-602 was well tolerated with one pt with DLT noted at the 50 mg/kg weekly dose level. Notably, all 3 pts treated at this dose level had evidence of clinical activity with 2/3 pts achieving MRD negative CR.
Click to Show/Hide
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Patients Enrolled |
R/R B-acute lymphocytic leukemia (ALL).
|
||||
| Administration Dosage |
A 3+3 dose-escalation design was used for phase 1. ADCT-602 was initially given IV once every 3 weeks (30 ug/kg, n=3; 60 ug/kg, n=4; 90 ug/kg, n=4); based on the PK data, the administration schedule was later amended to weekly infusions (30 ug/kg, n=3; 40 ug/kg, n=4; 50 ug/kg, n=3).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03698552 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/2 study to evaluate the safety and anti-tumor activity of ADCT-602 targeting CD22 in patients with relapsed or refractory b-cell acute lymphoblastic leukemia. | ||||
| Primary Endpoint |
In this phase 1 study in pts with very heavily pretreated R/R B-ALL with a median of 5 prior lines of therapy and high baseline bone marrow tumor burden, single-agent ADCT-602 was well tolerated with no DLTs noted. Two pts achieved MRD-negative remission. Dose escalation in the weekly schedule continues and 2 additional dose levels (40 ug/kg weekly and 50 ug/kg weekly) are planned. PK data, available for 9 pts treated at every 3-week schedule [30 ug/kg, n=3; 60 ug/kg, n=4; 90 ug/kg, n=2] showed rapid clearance of antibody with mean apparent half-life of <1 day during Cycle 1. This supported transitioning ADCT-602 administration to the weekly dosing.
Click to Show/Hide
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03698552 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/2 study to evaluate the safety and anti-tumor activity of ADCT-602 targeting CD22 in patients with relapsed or refractory B-cell acute lymphoblastic leukemia. | ||||
References
