Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0VBVDR
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
ADCT-502
|
|||||
| Synonyms |
ADCT 502; ADCT-502; ADCT502
Click to Show/Hide
|
|||||
| Organization |
ADC Therapeutics SA
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
1.7
|
|||||
| Structure |
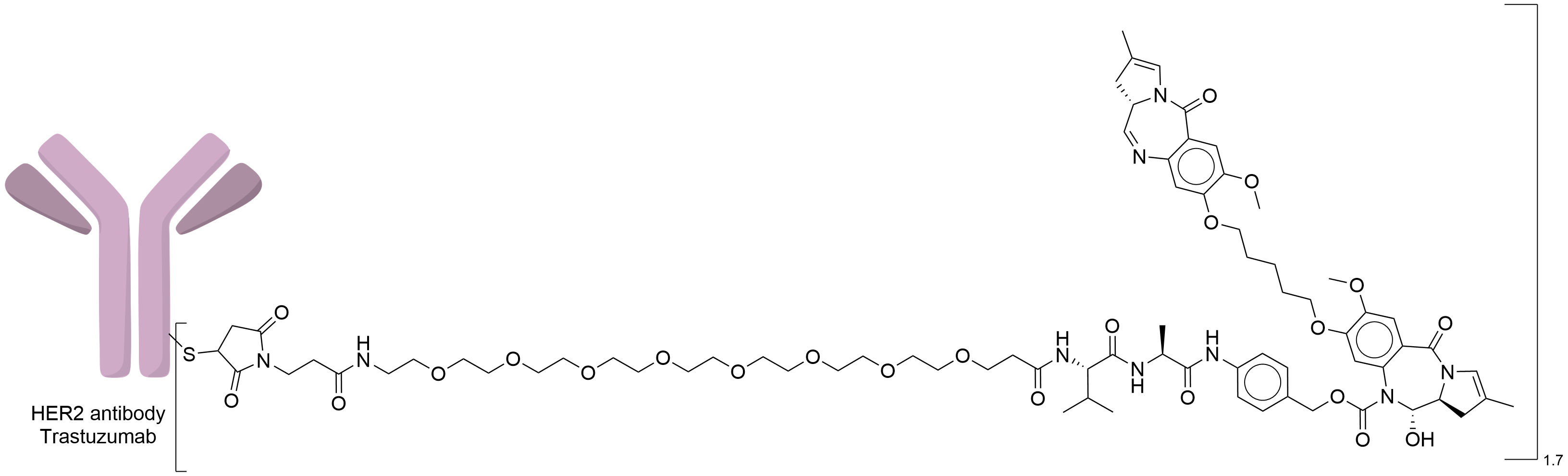
|
|||||
| Antibody Name |
Trastuzumab
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
SG3199
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
Mal-PEG8-Val-Ala-PABC
|
Linker Info | ||||
| Conjugate Type |
Reactive Cysteines
|
|||||
| Combination Type |
tesirine
|
|||||
| Puchem SID | ||||||
| TTD ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
20.00% (150 ug/kg)
|
|||
| Patients Enrolled |
Patients with HER2-positive advanced solid tumors.
|
||||
| Administration Dosage |
Seven dose cohorts (30, 60, 120, 150, 180, 210, 240 ug/kg on day 1, iv once every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03125200 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, open-label, dose-escalation study to evaluate the safety, tolerability, pharmacokinetics, and antitumor activity of ADCT-502 in patients with advanced solid tumors with HER2 expression. | ||||
References
