Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0PQJDH
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
MEDI-2228
|
|||||
| Synonyms |
MEDI 2228; MEDI-2228; MEDI2 228; MEDI2228
Click to Show/Hide
|
|||||
| Organization |
MedImmune LLC; AstraZeneca PLC
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
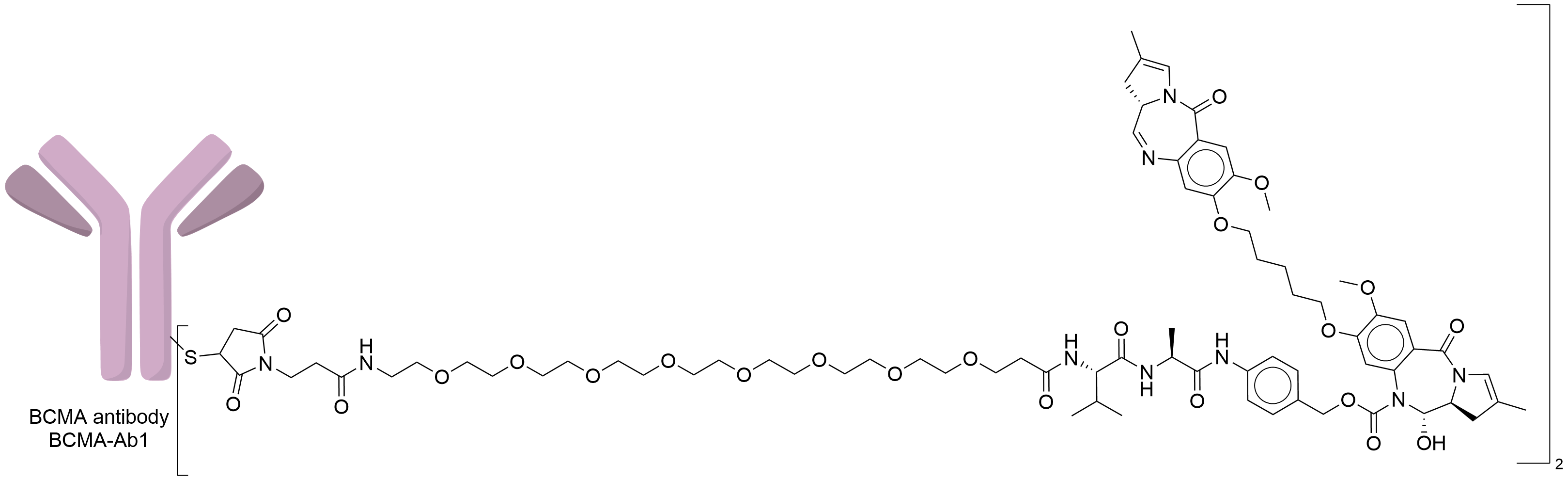
|
|||||
| Antibody Name |
BCMA-Ab1
|
Antibody Info | ||||
| Antigen Name |
Tumor necrosis factor receptor superfamily member 17 (TNFRSF17)
|
Antigen Info | ||||
| Payload Name |
SG3199
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
Mal-PEG8-Val-Ala-PABC
|
Linker Info | ||||
| Conjugate Type |
Reactive Cysteines
|
|||||
| Combination Type |
tesirine
|
|||||
| Puchem SID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Cell Line-derived Xenograft Model
Obtained from the Model Organism Data
| Standard Type | Value | Units | Animal Model (No. of PDX) |
|---|---|---|---|
| Tumor Growth Inhibition value (TGI) |
≈ 67.99
|
%
|
CB17 SCID mice model
|
| Tumor Growth Inhibition value (TGI) |
≈ 90.12
|
%
|
CB17 SCID mice model
|
Revealed Based on the Cell Line Data
| Standard Type | Value | Units | Cell Line | Disease Model |
|---|---|---|---|---|
| Half Maximal Effective Dose (EC50) |
189.7
|
ng/mL
|
RPMI-8226 cells
|
Plasma cell myeloma
|
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
61.00% (MTD)
|
|||
| Patients Enrolled |
Eligible pts were 18 years old with confirmed relapsed/refractory multiple myeloma (R/R MM) as defined by International Myeloma Working Group consensus criteria, had measurable disease, and had an Eastern Cooperative Oncology Group performance status 1. Pts had to have progressed after treatment with three classes of standard-of-care anti-myeloma drugs, including proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), and monoclonal antibodies (mAbs).
Click to Show/Hide
|
||||
| Administration Dosage |
Administered in the dose of 0.0125-0.20 mg/kg intravenously every 3 weeks (Q3W); The maximum tolerated dose was 0.14 mg/kg Q3W.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03489525 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, open-label study to evaluate the safety, pharmacokinetics, immunogenicity, and preliminary efficacy of MEDI2228 in subjects with relapsed/refractory multiple myeloma. | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 82.05% (Day 31) | |||
| Method Description |
The inhibitory activity of MEDI-2228 against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.3 mg/kg MEDI-2228.
|
||||
| In Vivo Model | NSG mice model | ||||
| In Vitro Model | Plasma cell myeloma | MM1.S cells | CVCL_8792 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.46% (Day 31) | |||
| Method Description |
The inhibitory activity of MEDI-2228+NK against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.3 mg/kg MEDI-2228.
|
||||
| In Vivo Model | NSG mice model | ||||
| In Vitro Model | Plasma cell myeloma | MM1.S cells | CVCL_8792 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.94% (Day 31) | |||
| Method Description |
The inhibitory activity of MEDI-2228+NK+Dara against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 0.3 mg/kg MEDI-2228.
|
||||
| In Vivo Model | NSG mice model | ||||
| In Vitro Model | Plasma cell myeloma | MM1.S cells | CVCL_8792 | ||
Obtained from the Model Organism Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 67.99% (Day 24) | |||
| Method Description |
The inhibitory activity of MEDI-2228 against cancer cell growth was evaluated in various human cancer cell lines in vivo.The cells were treated with 0.4 mg/kg MEDI-2228.
|
||||
| In Vivo Model | CB17 SCID mice model | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.12% (Day 24) | |||
| Method Description |
The inhibitory activity combination of M2 and btz for 2 weeks against cancer cell growth was evaluated in various human cancer cell lines in vivo.
|
||||
| In Vivo Model | CB17 SCID mice model | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Effective Dose (EC50) |
189.70 ng/mL
|
|||
| Method Description |
The inhibitory activity of MEDI-2228 against cancer cell growth was evaluated in various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Plasma cell myeloma | RPMI-8226 cells | CVCL_0014 | ||
References
