Payload Information
General Information of This Payload
| Payload ID | PAY0GTSVM |
|||||
|---|---|---|---|---|---|---|
| Name | Mertansine DM4 |
|||||
| Synonyms |
DM4; 796073-69-3; WOB38VS2NI; UNII-WOB38VS2NI; Ravtansine; DM 4; DM-4; (14S,16S,32S,33S,2R,4S,10E,12E,14R)-86-chloro-14-hydroxy-85,14-dimethoxy-33,2,7,10-tetramethyl-12,6-dioxo-7-aza-1(6,4)-oxazinana-3(2,3)-oxirana-8(1,3)-benzenacyclotetradecaphane-10,12-dien-4-yl N-(4-mercapto-4-methylpentanoyl)-N-methyl-L-alaninate; N2'-Deacetyl-N2'-(4-mercapto-4-methyl-1-oxopentyl)-maytansine; Maytansinoid DM 4; SCHEMBL12719514; EX-A3441; CS-6866; AC-36376; HY-12454; D94659; Q27292749; MAYTANSINE, N2'-DEACETYL-N2'-(4-MERCAPTO-4-METHYL-1-OXOPENTYL); [(1S,2R,3S,5S,6S,16E,18E,20R,21S)-11-Chloro-21-hydroxy-12,20-dimethoxy-2,5,9,16-tetramethyl-8,23-dioxo-4,24-dioxa-9,22-diazatetracyclo[19.3.1.110,14.03,5]hexacosa-10,12,14(26),16,18-pentaen-6-yl] (2S)-2-[methyl-(4-methyl-4-sulfanylpentanoyl)amino]propanoate
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
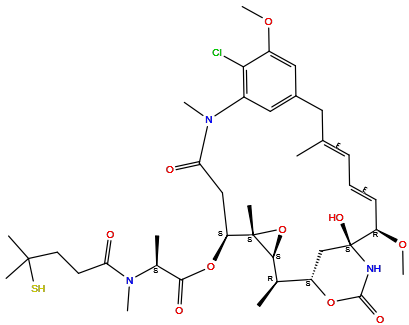
|
|||||
| Formula | C38H54ClN3O10S |
|||||
| Isosmiles | [H]O[C@@]12N([H])C(=O)O[C@@]([H])(C1([H])[H])[C@@]([H])(C([H])([H])[H])[C@]1([H])O[C@@]1(C([H])([H])[H])[C@@]([H])(OC(=O)[C@@]([H])(N(C(=O)C([H])([H])C([H])([H])C(S[H])(C([H])([H])[H])C([H])([H])[H])C([H])([H])[H])C([H])([H])[H])C([H])([H])C(=O)N(C([H])([H])[H])c1c([H])c(c([H])c(OC([H])([H])[H])c1Cl)C([H])([H])/C(C([H])([H])[H])=C([H])/C([H])=C(\[H])[C@@]2([H])OC([H])([H])[H] |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C38H54ClN3O10S/c1-21-12-11-13-28(49-10)38(47)20-27(50-35(46)40-38)22(2)33-37(6,52-33)29(51-34(45)23(3)41(7)30(43)14-15-36(4,5)53)19-31(44)42(8)25-17-24(16-21)18-26(48-9)32(25)39/h11-13,17-18,22-23,27-29,33,47,53H,14-16,19-20H2,1-10H3,(H,40,46)/b13-11+,21-12+/t22-,23+,27+,28-,29+,33+,37+,38+/m1/s1
|
|||||
| InChIKey |
JFCFGYGEYRIEBE-YVLHJLIDSA-N
|
|||||
| IUPAC Name |
[(1S,2R,3S,5S,6S,16E,18E,20R,21S)-11-chloro-21-hydroxy-12,20-dimethoxy-2,5,9,16-tetramethyl-8,23-dioxo-4,24-dioxa-9,22-diazatetracyclo[19.3.1.110,14.03,5]hexacosa-10,12,14(26),16,18-pentaen-6-yl] (2S)-2-[methyl-(4-methyl-4-sulfanylpentanoyl)amino]propanoate
|
|||||
| Pharmaceutical Properties | Molecule Weight |
780.381 |
Polar area |
156.47 |
||
Complexity |
779.3218436 |
xlogp Value |
5.0031 |
|||
Heavy Count |
53 |
Rot Bonds |
20 |
|||
Hbond acc |
11 |
Hbond Donor |
3 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 1 | nM |
Hep-G2 cells
|
Hepatoblastoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 10 | nM |
Lu1 cells
|
Lung carcinoma
|
Undisclosed | [2] |
| Half Maximal Effective Concentration (EC50) | 20 | nM |
Hep-G2 cells
|
Hepatoblastoma
|
[3] | |
| Half Maximal Effective Concentration (EC50) | 510 | nM |
COS-1 cells
|
Normal
|
[3] | |
| Half Maximal Effective Concentration (EC50) | 570 | nM |
Hep-G2 cells
|
Hepatoblastoma
|
[4] | |
| Half Maximal Effective Concentration (EC50) | 75 | nM |
HEK293 cells
|
Normal
|
[5] |
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
Mirvetuximab soravtansine [Approved]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
22.00%
|
High FOLR1 expression (FOLR1+++) | ||
| Patients Enrolled |
Platinum-resistant epithelial ovarian cancer (EOC); Eligible patients with 1-3 prior lines of therapy and whose tumors were positive for FR expression.
|
||||
| Administration Dosage |
6 mg/kg Q3W.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02631876 | Phase Status | Phase 3 | ||
| Clinical Description |
FORWARD I: A randomized, open label phase 3 study to evaluate the safety and efficacy of mirvetuximab soravtansine (IMGN853) versus investigator's choice of chemotherapy in women with folate receptor alpha positive advanced epithelial ovarian cancer, primary peritoneal cancer or fallopian tube cancer.
|
||||
| Primary Endpoint |
For the ITT population, Kaplan-Meier estimates showed no significant difference (HR, 0.98; 95% Cl, 0.73-1.31; P=0.897) in progression-free survival (PFS) between groups Median PFS was 4.10 and 4.40 months for MIRV and IC chemotherapy, respectively. In the prespecified high FR subgroup, PFS was longer in patients in the MIRV group compared with IC chemotherapy (median, 4.80 months versus 3.30 months; HR, 0.69, 95% CI, 0.48 to 1.00; P = 0.049). Based on the Hochberg procedure used in the statistical analysis plan for the study, this P value did not meet statistical significanceBoth in the ITT group and high FR subgroup, OS were not significantly different.
Click to Show/Hide
|
||||
| Other Endpoint |
Mirvetuximab soravtansine vs IC chemotherapy in the ITT group: ORR=22.00% vs 12.00%, P=0.015; cancer antigen-125 (CA-125) responses=51.00% vs 27.00%, P<0001; median PFS2 duration (time from randomization to second disease progression or death)=10.00 vs 8.40 months, HR=0.64, 95% Cl, 0.49-0.84, P<0.001); PRO in the EORTC QLQ-OV28 Abdominal/GI Symptom Subscale=32.00% vs 14.00%, P=0.016; Mirvetuximab soravtansine vs IC chemotherapy in the high FR subset, ORR=24.00% vs 10.00%, P=0.014; cancer antigen-125 (CA-125) responses=53.00% vs 25.00%, P=0.001; median PFS2 duration=10.10 vs 8.40 months, HR=0.56, 95% Cl, 0.39-0.79, P<0.001); PRO in the EORTC QLQ-OV28 Abdominal/GI Symptom Subscale=27.00% vs 13.00%, P=0.143.
Click to Show/Hide
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
31.73%
|
High FOLR1 expression (FOLR1+++) | ||
| Patients Enrolled |
Platinum-Resistant, Advanced High-Grade Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancers With High Folate Receptor-Alpha Expression.
|
||||
| Administration Dosage |
6 mg/kg Q3W.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT04296890 | Phase Status | Phase 3 | ||
| Clinical Description |
SORAYA: A Phase 3, single arm study of mirvetuximab soravtansine in platinum-resistant, advanced high-grade epithelial ovarian, primary peritoneal, or fallopian tube cancers with high folate receptor-alpha expression.
|
||||
| Primary Endpoint |
Confirmed Overall Response Rate=31.73% [(N=104, 95% Cl, 22.90-41.60), Complete response rate=4.80%, Partial response rate=26.90%].
|
||||
| Other Endpoint |
Median duration of response=6.90 months (N=33, 95% Cl 5.60-9.70).
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
39.00%
|
Moderate FOLR1 expression (FOLR1++) | ||
| Patients Enrolled |
Platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer.
|
||||
| Administration Dosage |
6 mg/kg (adjusted ideal body weight) once every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02606305 | Phase Status | Phase 1b/2 | ||
| Clinical Description |
A phase 1b/2 study to evaluate the safety, tolerability and pharmacokinetics of mirvetuximab soravtansine (IMGN853) in combination with bevacizumab, carboplatin, pegylated liposomal doxorubicin, pembrolizumab, or bevacizumab + carboplatin, in adults with folate receptor alpha positive advanced epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer.
Click to Show/Hide
|
||||
| Primary Endpoint |
For AURELIA-type patients (N=16, bevacizumab-naive and 1-2 prior lines of therapy, plus medium/high FRa expression), confirmed ORR=56.00%, median duration of response (mDOR)=12.0 months, median progression-free survival (mPFS) = 9.9 months.
|
||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.40% (Day 30) | Moderate ROR1 expression (ROR1++) | ||
| Method Description |
In vivoexperiments comparing the antitumor activity of IMGN853 versus ADC isotype control, PBS and M9346A were conducted using both high grade endometrioid/clear cell xenografts as well as a uterine serous tumor PDX model (BIO (K)1).all treatments were given twice, one week apart by retro-orbital injection at a concentration of 5 mg/kg.
|
||||
| In Vivo Model | Uterine serous carcinomas PDX model (PDX: BIO(K)1) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 25) | Moderate ROR1 expression (ROR1++) | ||
| Method Description |
In vivoexperiments comparing the antitumor activity of IMGN853 versus ADC isotype control, PBS and M9346A were conducted using both high grade endometrioid/clear cell xenografts as well as a uterine serous tumor PDX model (BIO (K)1).all treatments were given twice, one week apart by retro-orbital injection at a concentration of 5 mg/kg.
|
||||
| In Vivo Model | Uterine serous carcinomas PDX model (PDX: END(K)265) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 33.00% (Day 5) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OV-90 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 25 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OV-90 CDX model | ||||
| In Vitro Model | Ovarian adenocarcinoma | OV-90 cells | CVCL_3768 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.00% (Day 6) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of IGROV-1 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 25 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | IGROV-1 CDX model | ||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 64.00% (Day 26) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OV-90 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 50 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OV-90 CDX model | ||||
| In Vitro Model | Ovarian adenocarcinoma | OV-90 cells | CVCL_3768 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 82.00% (Day 40) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OV-90 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 100 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OV-90 CDX model | ||||
| In Vitro Model | Ovarian adenocarcinoma | OV-90 cells | CVCL_3768 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 82.00% (Day 21) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OVCAR-3 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 25 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
93.00% (Day 32)
|
High FOLR1 expression (FOLR1+++; 1,300,000 FOLR1 molecules/cell) | ||
| Method Description |
Animals with established tumors of about 130 mm3 were treated with intravenous single injection of the M9346A-DM conjugates at 2.5±0.2 mg/kg, equivalent to 51±3 ug conjugated maytansinoid per kg The conjugates were injected on day 7 after cell inoculation.
|
||||
| In Vivo Model | Ovarian carcinoma Igrov-1 CDX model | ||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.00% (Day 41) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of IGROV-1 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 50 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | IGROV-1 CDX model | ||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.00% (Day 67) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of IGROV-1 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 100 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | IGROV-1 CDX model | ||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
99.07% (Day 30)
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Animals with established tumors of about 130 mm3 were treated with intravenous single injection of the M9346A-DM conjugates at 2.5±0.2 mg/kg, equivalent to 51±3 ug conjugated maytansinoid per kg The conjugates were injected on day 20 after cell inoculation.
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 75) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OVCAR-3 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 50 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 120) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OVCAR-3 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 100 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.15±0.08 nM
|
|||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.20±0.10 nM
|
|||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Gestational choriocarcinoma | JEG-3 cells | CVCL_0363 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00±0.40 nM
|
|||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.20 ug/mL
|
Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
Cell cytotoxicity was tested with IMGN853, ADC isotype control and M9346A in pure and mixed endometrial endometrioid cell lines harboring high FOLR1 expression.
|
||||
| In Vitro Model | Endometrial undifferentiated carcinoma | END-2 cells | CVCL_B5KE | ||
| Experiment 5 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
74.60 ng/mL
|
Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
Cell cytotoxicity was tested with IMGN853, ADC isotype control and M9346A in pure and mixed endometrial endometrioid cell lines harboring high FOLR1 expression.
|
||||
| In Vitro Model | Endometrial undifferentiated carcinoma | END-1 cells | CVCL_B5JE | ||
Tusamitamab ravtansine [Phase 3]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
40.00%
|
Low CEACAM5 expression (CEACAM5+; 299,300 sites/cell) | ||
| Patients Enrolled |
Patients with advanced/metastatic nonsquamous non-small cell lung cancer (NSQ NSCLC) with CEACAM5 intensity of 2+ in 1% of tumor cells by immunohistochemistry.
|
||||
| Administration Dosage |
IV Q3W at 150 or 170 mg/m2.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT04524689 | Phase Status | Phase 2 | ||
| Clinical Description |
Open-label, phase 2 study of tusamitamab ravtansine (SAR408701) combined with pembrolizumab and tusamitamab ravtansine (SAR408701) combined with pembrolizumab and platinum-based chemotherapy with or without pemetrexed in patients with CEACAM5 positive expression advanced/metastatic non-squamous non-small-cell lung cancer (nsq NSCLC).
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
20.30% (CEACAM5 High-expression)
7.10% (CEACAM5 Moderate-expression) 41.70% (high cCEA) 8.10% (Low cCEA) |
Moderate CEACAM5 expression (CEACAM5++; 1,615,700 sites/cell) | ||
| Patients Enrolled |
Enrolled 2 cohorts of patients with IHC CEACAM5 membrane expression at 2+ intensity: in 50% of tumor cells (high expressors, HEs, n = 64); and in 1% to <50% of tumor cells (moderate expressors, MEs, n = 28).
|
||||
| Administration Dosage |
100 mg/m2 IV every 2 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02187848 | Phase Status | Phase 1 | ||
| Clinical Description |
A first-in-human study for the evaluation of the safety, pharmacokinetics and antitumor activity of SAR408701 in patients with advanced solid tumors.
|
||||
| Primary Endpoint |
The primary endpoint was the incidence of DLTs occurring during the first two cycles (4 weeks) of study drug administration. DLTs (reversible grade 3 microcystic keratopathy) occurred in three of eight patients treated with tusamitamab ravtansine 12.00 mg/m2 and in two of three patients treated with 15.00 mg/m2. The maximum tolerated dose was identified as 10.00 mg/m2.
Click to Show/Hide
|
||||
| Other Endpoint |
Three patients (9.68%) had objective responses [all confirmed partial responses (PRs) with durations of 2.60, 6.10, and 4.00 months]; 11 patients (35.48%) had stable disease, and 13 patients (41.94%) had progressive disease. Objective responses were achieved in two of six patients (33.33%) at a DL of 10.0 mg/m2, and in one of nine patients (11.11%) at 12.0 mg/m2 with maximum reduction in RECIST target lesions of 32.30%-51.20%.
Click to Show/Hide
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [14] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05703555 | Phase Status | Phase 2 | ||
| Clinical Description |
Intrusion: unraveling the intratumoral PK/PD relation for SAR408701.
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [15] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05245071 | Phase Status | Phase 2 | ||
| Clinical Description |
Open-label, phase 2 study, evaluating the efficacy and safety of tusamitamab ravtansine in non-squamous non-small-cell lung cancer (nsq NSCLC) participants with negative or moderate CEACAM5 expression tumors and high circulating CEA.
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [16] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05071053 | Phase Status | Phase 2 | ||
| Clinical Description |
Open-label study of tusamitamab ravtansine (SAR408701) in combination with ramucirumab in participants previously treated for advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma with CEACAM5-positive tumors.
|
||||
| Experiment 6 Reporting the Activity Date of This ADC | [17] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04659603 | Phase Status | Phase 2 | ||
| Clinical Description |
Open-label, multi-cohort, phase 2 trial, evaluating the efficacy and safety of tusamitamab ravtansine (SAR408701) monotherapy and in combination in patients with CEACAM5-positive advanced solid tumors.
|
||||
| Experiment 7 Reporting the Activity Date of This ADC | [18] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05429762 | Phase Status | Phase 1 | ||
| Clinical Description |
Open-label study evaluating the effect of tusamitamab ravtansine on the QTC interval in participants with metastatic solid tumors.
|
||||
| Experiment 8 Reporting the Activity Date of This ADC | [19] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04154956 | Phase Status | Phase 1 | ||
| Clinical Description |
Randomized, open-label, phase 3 study of SAR408701 versus docetaxel in previously treated, metastatic nonsquamous, non-small-cell lung cancer patients with CEACAM5-positive tumors.
|
||||
| Experiment 9 Reporting the Activity Date of This ADC | [20] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03324113 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 study to evaluate safety and pharmacokinetics of SAR408701 administered intravenously as monotherapy in japanese patients with advanced malignant solid tumors.
|
||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
0.00% (Day 28)
|
Moderate CEACAM5 expression (CEACAM5++; IHC 2+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a lung adenocarcinoma patient with IHC 2+, with a single intravenous administration at 2.5 mg/kg.
|
||||
| In Vivo Model | Lung adenocarcinoma PDX model (PDX: LUN-NIC-014) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
6.40% (Day 28)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, with a single intravenous administration at 2.5 mg/kg.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-002C/M) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
27.10% (Day 28)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a stomach adenocarcinoma patient with IHC 3+, with a single intravenous administration at 2.5 mg/kg.
|
||||
| In Vivo Model | Stomach adenocarcinoma PDX model (PDX: STO-IND-0007) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
32.90% (Day 28)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, with a single intravenous administration at 5 mg/kg.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-002C/M) | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
41.50% (Day 17)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a stomach adenocarcinoma patient with IHC 3+, with a single intravenous administration at 2.5 mg/kg.
|
||||
| In Vivo Model | Stomach adenocarcinoma PDX model (PDX: SA-STO-0014) | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
55.00% (Day 28)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a stomach adenocarcinoma patient with IHC 3+, twice a week with a single intravenous administration at 2.5 mg/kg for a total of 4 weeks.
|
||||
| In Vivo Model | Stomach adenocarcinoma PDX model (PDX: STO-IND-0007) | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
57.20% (Day 17)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a stomach adenocarcinoma patient with IHC 3+, with a single intravenous administration at 5 mg/kg.
|
||||
| In Vivo Model | Stomach adenocarcinoma PDX model (PDX: SA-STO-0014) | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
60.30% (Day 28)
|
Moderate CEACAM5 expression (CEACAM5++; IHC 2+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a lung adenocarcinoma patient with IHC 2+, with a single intravenous administration at 2.5 mg/kg.
|
||||
| In Vivo Model | Lung adenocarcinoma PDX model (PDX: LUN-NIC-0083) | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
63.90% (Day 28)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, with a single intravenous administration at 2.5 mg/kg.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-0034P) | ||||
| Experiment 10 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
66.20% (Day 28)
|
Moderate CEACAM5 expression (CEACAM5++; IHC 2+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a lung adenocarcinoma patient with IHC 2+, with a single intravenous administration at 5 mg/kg.
|
||||
| In Vivo Model | Lung adenocarcinoma PDX model (PDX: LUN-NIC-0083) | ||||
| Experiment 11 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
69.70% (Day 28)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a stomach adenocarcinoma patient with IHC 3+, with a single intravenous administration at 5 mg/kg.
|
||||
| In Vivo Model | Stomach adenocarcinoma PDX model (PDX: STO-IND-0007) | ||||
| Experiment 12 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
69.80% (Day 28)
|
Moderate CEACAM5 expression (CEACAM5++; IHC 2+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a lung adenocarcinoma patient with IHC 2+, with a single intravenous administration at 10 mg/kg.
|
||||
| In Vivo Model | Lung adenocarcinoma PDX model (PDX: LUN-NIC-0083) | ||||
| Experiment 13 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
70.20% (Day 28)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a stomach adenocarcinoma patient with IHC 3+, with a single intravenous administration at 5 mg/kg.
|
||||
| In Vivo Model | Stomach adenocarcinoma PDX model (PDX: STO-IND-0007) | ||||
| Experiment 14 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
71.80% (Day 28)
|
Moderate CEACAM5 expression (CEACAM5++; IHC 2+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a lung adenocarcinoma patient with IHC 2+, with a single intravenous administration at 5 mg/kg.
|
||||
| In Vivo Model | Lung adenocarcinoma PDX model (PDX: LUN-NIC-014) | ||||
| Experiment 15 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
76.50% (Day 28)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a stomach adenocarcinoma patient with IHC 3+, once a week with a single intravenous administration at 5 mg/kg for a total of 4 weeks.
|
||||
| In Vivo Model | Stomach adenocarcinoma PDX model (PDX: STO-IND-0007) | ||||
| Experiment 16 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
84.70% (Day 28)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, with a single intravenous administration at 5 mg/kg.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-0034P) | ||||
| Experiment 17 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
90.20% (Day 28)
|
Moderate CEACAM5 expression (CEACAM5++; IHC 2+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a lung adenocarcinoma patient with IHC 2+, with a single intravenous administration at 10 mg/kg.
|
||||
| In Vivo Model | Lung adenocarcinoma PDX model (PDX: LUN-NIC-014) | ||||
| Experiment 18 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
90.30% (Day 28)
|
Moderate CEACAM5 expression (CEACAM5++; IHC 2+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a lung adenocarcinoma patient with IHC 2+, twice a week with a single intravenous administration at 2.5 mg/kg for a total of 4 weeks.
|
||||
| In Vivo Model | Lung adenocarcinoma PDX model (PDX: LUN-NIC-014) | ||||
| Experiment 19 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
91.40% (Day 28)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, with a single intravenous administration at 10 mg/kg.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-002C/M) | ||||
| Experiment 20 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
92.40% (Day 28)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a stomach adenocarcinoma patient with IHC 3+, with a single intravenous administration at 10 mg/kg.
|
||||
| In Vivo Model | Stomach adenocarcinoma PDX model (PDX: STO-IND-0007) | ||||
| Experiment 21 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
92.80% (Day 28)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, with a single intravenous administration at 10 mg/kg.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-0034P) | ||||
| Experiment 22 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
92.80% (Day 28)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, with a single intravenous administration at 5 mg/kg.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-0034P) | ||||
| Experiment 23 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
93.30% (Day 17)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a stomach adenocarcinoma patient with IHC 3+, with a single intravenous administration at 10 mg/kg.
|
||||
| In Vivo Model | Stomach adenocarcinoma PDX model (PDX: SA-STO-0014) | ||||
| Experiment 24 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
93.50% (Day 28)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, with a single intravenous administration at 5 mg/kg.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-002C/M) | ||||
| Experiment 25 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
97.00% (Day 28)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, once a week with a single intravenous administration at 5 mg/kg for a total of 4 weeks.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-0034P) | ||||
| Experiment 26 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
99.40% (Day 28)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, once a week with a single intravenous administration at 5 mg/kg for a total of 4 weeks.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-002C/M) | ||||
| Experiment 27 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
99.90% (Day 28)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, twice a week with a single intravenous administration at 2.5 mg/kg for a total of 4 weeks.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-002C/M) | ||||
| Experiment 28 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 28)
|
High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, twice a week with a single intravenous administration at 2.5 mg/kg for a total of 4 weeks.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-0034P) | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.20±0.04 nM
|
Low CEACAM5 expression (CEACAM5+; 498,900 sites/cell) | ||
| Method Description |
The inhibitory activity of SAR408377 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated incubated overnight.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.38±0.07 nM
|
|||
| Method Description |
The inhibitory activity of SAR408377 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated incubated overnight.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.08±0.17 nM
|
|||
| Method Description |
The inhibitory activity of SAR408377 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated incubated overnight.
|
||||
| In Vitro Model | Gastric adenocarcinoma | MKN45 cells | CVCL_0434 | ||
Praluzatamab ravtansine [Phase 2]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [22] | ||||
| Efficacy Data | Partial Response (PR) |
9.00%
|
|||
| Patients Enrolled |
Eastern Cooperative Oncology Group (ECOG) 0, 1, metastatic or locally advanced unresectable solid tumors with progressive disease (PD) after standard treatment or known intolerance to available treatment, based on the predicted prevalence of CD166 expression, were breast cancer, castration-resistant prostate cancer, nonsmall cell lung cancer (NSCLC), epithelial ovarian cancer, head and neck squamous cell cancer (HNSCC), cholangiocarcinoma, and endometrial carcinoma.
Click to Show/Hide
|
||||
| Administration Dosage |
Scalating doses every 3 weeks (0.25-10 mg/kg) or every 2 weeks (4-6 mg/kg), IV.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03149549 | Phase Status | Phase 1/2 | ||
| Clinical Description |
A phase 1-2, open-label, dose-finding, proof of concept, first-in-human study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of CX-2009 in adults with metastatic or locally advanced unresectable solid tumors (PROCLAIM-CX-2009).
|
||||
| Primary Endpoint |
Median number of prior therapies was 5. On the basis of tolerability, the RP2D was 7 mg/kg every 3 weeks. Tumor regressions were observed at doses 4 mg/kg.
|
||||
Anetumab ravtansine [Phase 2 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [23] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
9.60%
|
Moderate MSLN expresion (MSLN++; 135,000-480,000 CD48 molecules/cell) | ||
| Patients Enrolled |
Unresectable locally advanced or metastatic malignant pleural mesothelioma, an Eastern Cooperative Oncology Group performance status of 0-1, and who had progressed on first-line platinum-pemetrexed chemotherapy with or without bevacizumab.
|
||||
| Administration Dosage |
Anetumab ravtansine (6.50 mg/kg once every 3 weeks) via intravenous infusion for 1 h on day 1 of each 21-day cycle, or vinorelbine (30 mg/m2 once every week) via intravenous injection for 610 min.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02610140 | Phase Status | Phase 2 | ||
| Clinical Description |
A randomized, open-label, active-controlled, phase 2 study of intravenous anetumab ravtansine (BAY 94-9343) or vinorelbine in patients with advanced or metastatic malignant pleural mesothelioma overexpressing mesothelin and progressed on first line platinum/pemetrexed-based chemotherapy.
|
||||
| Primary Endpoint |
For 6.50 mg/kg anetumab ravtansine,median progression-free survival 4.30 months [95% CI 41.00-52.00].
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [24] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
27.70% (all treated patients)
42.10% (In high mesothelin expression patients(N=19) who received 3 prior lines of systemic therapy) |
High MSLN expresion (MSLN+++; 19,998 MSLN molecules/cell) | ||
| Patients Enrolled |
Predominantly epithelial (>50% of tumor component) platinum-resistant recurrent ovarian, fallopian tube, or primary peritoneal cancer.
|
||||
| Administration Dosage |
Anetumab ravtansine (5.50 or 6.50 mg/kg) and pegylated liposomal doxorubicin (30 mg/m2) were administered intravenously every 3 weeks to 65 patients with platinum-resistant epithelial ovarian cancer.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02751918 | Phase Status | Phase 1b | ||
| Clinical Description |
An open-label phase 1b dose escalation study to evaluate the safety, tolerability, pharmacokinetics, immunogenicity and maximum tolerated dose of anetumab ravtansine in combination with pegylated liposomal doxorubicin 30 mg/m<sup>2</sup> given every 3 weeks in subjects with mesothelin-expressing platinum-resistant recurrent ovarian, fallopian tube or primary peritoneal cancer.
Click to Show/Hide
|
||||
| Primary Endpoint |
The maximum tolerated dose of anetumab ravtansine in combination was 6.50 mg/kg administered every 3 weeks. No patient experienced a dose-limiting toxicity at either dose in the dose escalation cohort.
|
||||
| Other Endpoint |
In all treated patients, ORR=27.70% (95% CI 17.30% to 40.20%), including one complete (1.50%) and 17 partial responses (26.20%). Mdor=7.60 months (95% CI 3.30 to 10.20), mPFS=5.0 months (95% CI 3.20 to 6.00). In high mesothelin expression patients (N=19), who received 3 prior lines of systemic therapy, ORR=42.10% (95% CI 20.30% to 66.50%), mDOR=8.30 months (95% CI 4.10 to 12.00), mFPS=8.50 months (95% CI 4.00 to 11.40).
Click to Show/Hide
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [25] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
31.00% (6.5 mg/kg, Mesothelioma)
|
Moderate MSLN expresion (MSLN++; 1105 MSLN molecules/cell) | ||
| Patients Enrolled |
Advanced, metastatic, or recurrent solid tumors refractory to standard therapy.
|
||||
| Administration Dosage |
0.15, 0.30, 0.60, 1.20, 2.40, 3.60, 4.50, 5.50, 6.50, and 7.50 mg/kg once every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01439152 | Phase Status | Phase 1 | ||
| Clinical Description |
An open label phase 1 dose escalation study to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics, and maximum tolerated dose of the anti-mesothelin antibody drug conjugate BAY94-9343 in subjects with advanced solid tumors.
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [27] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03926143 | Phase Status | Phase 2 | ||
| Clinical Description |
An open-label, multicenter rollover study to provide continued treatment with anetumab ravtansine for participants with solid tumors who were enrolled in previous bayer-sponsored studies.
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [28] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03587311 | Phase Status | Phase 2 | ||
| Clinical Description |
A randomized phase 2 study of bevacizumab and either weekly anetumab ravtansine or weekly paclitaxel in platinum-resistant or platinum refractory ovarian cancer.
|
||||
| Experiment 6 Reporting the Activity Date of This ADC | [29] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03023722 | Phase Status | Phase 2 | ||
| Clinical Description |
An open-label, phase 2 study of intravenous anetumab ravtansine (BAY 94-9343), an anti-mesothelin antibody drug conjugate, in pretreated mesothelin-expressing advanced pancreatic cancer.
|
||||
| Experiment 7 Reporting the Activity Date of This ADC | [30] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02839681 | Phase Status | Phase 2 | ||
| Clinical Description |
Phase 2 trial with safety run-in of the anti-mesothelin antibody drug conjugate anetumab ravtansine for mesothelin expressing lung adenocarcinoma.
|
||||
| Experiment 8 Reporting the Activity Date of This ADC | [31] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02610140 | Phase Status | Phase 2 | ||
| Clinical Description |
A randomized, open-label, active-controlled, phase 2 study of intravenous anetumab ravtansine (BAY 94-9343) or vinorelbine in patients with advanced or metastatic malignant pleural mesothelioma overexpressing mesothelin and progressed on first line platinum/pemetrexed-based chemotherapy.
|
||||
| Experiment 9 Reporting the Activity Date of This ADC | [32] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03126630 | Phase Status | Phase 1/2 | ||
| Clinical Description |
Phase 1 safety run-in and phase 2 randomized clinical trial of anetumab ravtansine and pembrolizumab (MK-3475) compared to pembrolizumab alone for mesothelin-positive malignant pleural mesothelioma.
|
||||
| Experiment 10 Reporting the Activity Date of This ADC | [37] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03816358 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 study of anetumab ravtansine in combination with either anti-PD-1 antibody, or anti-CTLA4 and anti-PD-1 antibodies or anti-PD-1 antibody and gemcitabine in mesothelin-positive advanced pancreatic adenocarcinoma.
|
||||
| Experiment 11 Reporting the Activity Date of This ADC | [38] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03455556 | Phase Status | Phase 1 | ||
| Clinical Description |
Phase 1/2 study of the human anti-mesothelin antibody drug conjugate anetumab ravtansine (AR), combined with the PD-L1 inhibitor atezolizumab in non-small cell lung cancer.
|
||||
| Experiment 12 Reporting the Activity Date of This ADC | [39] | ||||
| Patients Enrolled |
Unresectable locally advanced or metastatic recurrent or relapsing disease.
|
||||
| Administration Dosage |
Mesothelin-positive patients with selected adenocarcinomas (NSCLC, triple negative breast, gastric including gastroesophageal junction) and thymic carcinoma will receive anetumab ravtansine as monotherapy at 6.50 mg/kg IV on a 21-day cycle. Patients with cholangiocarcinoma will receive anetumab ravtansine in combination with cisplatin (25 mg/m2 IV day 1 and 8 on a 21-day cycle for up to 6 cycles) and patients with pancreatic adenocarcinoma will receive anetumab ravtansine in combination with gemcitabine (1000 mg/m2 IV day 1 and 8 on a 21-day cycle).
Click to Show/Hide
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03102320 | Phase Status | Phase 1 | ||
| Clinical Description |
Phase 1b multi-indication study of anetumab ravtansine (BAY94-9343) in patients with mesothelin expressing advanced or recurrent malignancies.
|
||||
| Experiment 13 Reporting the Activity Date of This ADC | [40] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02824042 | Phase Status | Phase 1 | ||
| Clinical Description |
An open label, phase 1 study to assess the effect of itraconazole (CYP3A4 and P-gp Inhibitor) on the pharmacokinetics of anetumab ravtansine and to assess the ECG Effects, safety and immunogenicity of anetumab ravtansine given as a single agent and together with itraconazole in subjects with mesothelin-expressing advanced solid cancers.
|
||||
| Experiment 14 Reporting the Activity Date of This ADC | [41] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02751918 | Phase Status | Phase 1 | ||
| Clinical Description |
An open-label phase 1b dose escalation study to evaluate the safety, tolerability, pharmacokinetics, immunogenicity and maximum tolerated dose of anetumab ravtansine in combination with pegylated liposomal doxorubicin 30 mg/m<sup>2</sup> given every 3 weeks in subjects with mesothelin-expressing platinum-resistant recurrent ovarian, fallopian tube or primary peritoneal cancer.
Click to Show/Hide
|
||||
| Experiment 15 Reporting the Activity Date of This ADC | [42] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02696642 | Phase Status | Phase 1 | ||
| Clinical Description |
An open label phase 1 study to evaluate the safety, tolerability, pharmacokinetics and immunogenicity of anetumab ravtansine in subjects with mesothelin-expressing advanced solid cancers and different stages of concurrent hepatic or renal impairment.
|
||||
| Experiment 16 Reporting the Activity Date of This ADC | [43] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02639091 | Phase Status | Phase 1 | ||
| Clinical Description |
An open label phase 1b dose escalation study to evaluate the safety, tolerability, pharmacokinetics, immunogenicity and maximum tolerated dose of anetumab ravtansine in combination with pemetrexed 500 mg/m<sup>2</sup> and cisplatin 75 mg/m<sup>2</sup> in subjects with mesothelin-expressing predominantly epithelial mesothelioma or nonsquamous non-small-cell lung cancer.
Click to Show/Hide
|
||||
| Experiment 17 Reporting the Activity Date of This ADC | [44] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02485119 | Phase Status | Phase 1 | ||
| Clinical Description |
An open label, phase 1 study to evaluate the safety, tolerability and pharmacokinetics of BAY94-9343 given by intravenous infusion every 3 weeks (Q3W) in Japanese subjects with advanced malignancies.
|
||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [45] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
0.00% (Day 70)
|
High Mesothelin expression (MSLN+++; IHC 3+) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive xenograft tumor models. Mice were subcutaneously inoculated with 5x106 MSLN positive T1889 cells mixed with Matrigel. Treatment then began, and the experiments ran for a total of 4 weeks. The first treatment cohort of 32 mice consisted of vehicle control, isotype ADC control, and 15 mg/kg ARav, dosed at Q7D via intraperitoneal injection. A second treatment cohort of 30 mice occurred with groups consisting of vehicle control (Q14D), 7.5 mg/kg ARav (Q14D) monotherapy, 7.5 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy. A third treatment cohort of 32 mice occurred with groups consisting of vehicle control (Q14D), 3.75 mg/kg ARav (Q14D) monotherapy, 3.75 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing cervical squamous cell carcinoma PDX model (PDX: Caski) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [45] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
0.00% (Day 70)
|
Moderate Mesothelin expression (MSLN++; IHC 2+) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive xenograft tumor models. Mice were subcutaneously inoculated with 5x106 MSLN positive T1889 cells mixed with Matrigel. Treatment then began, and the experiments ran for a total of 4 weeks. The first treatment cohort of 32 mice consisted of vehicle control, isotype ADC control, and 15 mg/kg ARav, dosed at Q7D via intraperitoneal injection. A second treatment cohort of 30 mice occurred with groups consisting of vehicle control (Q14D), 7.5 mg/kg ARav (Q14D) monotherapy, 7.5 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy. A third treatment cohort of 32 mice occurred with groups consisting of vehicle control (Q14D), 3.75 mg/kg ARav (Q14D) monotherapy, 3.75 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing uterine carcinosarcoma PDX model (PDX: Cx-03) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [46] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 27) | Moderate Mesothelin expression (MSLN++; IHC 2+) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive xenograft tumor models. Mice were subcutaneously inoculated with 5x106 MSLN positive T1889 cells mixed with Matrigel. Treatment then began, and the experiments ran for a total of 4 weeks. The first treatment cohort of 32 mice consisted of vehicle control, isotype ADC control, and 15 mg/kg ARav, dosed at Q7D via intraperitoneal injection. A second treatment cohort of 30 mice occurred with groups consisting of vehicle control (Q14D), 7.5 mg/kg ARav (Q14D) monotherapy, 7.5 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy. A third treatment cohort of 32 mice occurred with groups consisting of vehicle control (Q14D), 3.75 mg/kg ARav (Q14D) monotherapy, 3.75 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing uterine carcinosarcoma PDX model (PDX: T1889) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 16.70% (Day 153) | High Mesothelin expression (MSLN+++; IHC 3+) | ||
| Method Description |
For subcutaneous tumor models,female NMRI nu/nu mice (18-25 g,7-10 weeks) from Taconic M&B were implanted on day 0 with either 3 x106 MIA PaCa-2/vector,3 x106 MIA PaCa-2/meso,1 x106 HT-29/vector,1 x106 HT-29/meso,3 x106 OVCAR-3,or 3 x106 NCI-H226 cells suspended in 0.1 mL 50% Matrigel. Patient-derived pancreatic (PAXF736) model was performed at Oncotest GmbH and ovarian (OVCAR6719) and mesothelioma (Meso7212) models at EPO Berlin-Buch GmbH. 0.03 mg/kg , 0.05 mg/kg , 0.2 mg/kg Anetumab ravtansine was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing mesothelioma PDX model (PDX: NCI-H226) | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [45] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
25.00% (Day 70)
|
High Mesothelin expression (MSLN+++; IHC 3+) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive xenograft tumor models. Mice were subcutaneously inoculated with 5x106 MSLN positive T1889 cells mixed with Matrigel. Treatment then began, and the experiments ran for a total of 4 weeks. The first treatment cohort of 32 mice consisted of vehicle control, isotype ADC control, and 15 mg/kg ARav, dosed at Q7D via intraperitoneal injection. A second treatment cohort of 30 mice occurred with groups consisting of vehicle control (Q14D), 7.5 mg/kg ARav (Q14D) monotherapy, 7.5 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy. A third treatment cohort of 32 mice occurred with groups consisting of vehicle control (Q14D), 3.75 mg/kg ARav (Q14D) monotherapy, 3.75 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing uterine carcinosarcoma PDX model (PDX: Cx-03) | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
27.00%
|
Positive Mesothelin expression (MSLN+++/++) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing serous papillary carcinoma PDX model (PDX: ST206B) | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 35.10% (Day 28) | High Mesothelin expression (MSLN+++; IHC 3+) | ||
| Method Description |
For subcutaneous tumor models,female NMRI nu/nu mice (18-25 g, 7-10 weeks) from Taconic M&B were implanted on day 0 with either 3x106 MIA PaCa-2/vector,3x106 MIA PaCa-2/meso, 1x106 HT-29/vector, 1x106 HT-29/meso ,3x106 OVCAR-3, or 3x106 NCI-H226 cells suspended in 0.1 mL 50% Matrigel. Patient-derived pancreatic (PAXF736) model was performed at Oncotest GmbH and ovarian (OVCAR6719) and mesothelioma (Meso7212) models at EPO Berlin-Buch GmbH. 0.01 mg/kg , 0.05 mg/kg , 0.2 mg/kg Anetumab ravtansine was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing pancreatic carcinoma PDX model (PDX: MIA PaCa-2/meso) | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
42.00%
|
Positive Mesothelin expression (MSLN+++/++) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing high-grade serous ovarian cancer PDX model (PDX: OVCAR-8) | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
42.00%
|
Positive Mesothelin expression (MSLN+++/++) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing high-grade serous ovarian cancer PDX model (PDX: OVCAR-8) | ||||
| Experiment 10 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
49.00%
|
Moderate MSLN expression (MSLN++) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing high-grade serous ovarian cancer PDX model (PDX: ST081) | ||||
| Experiment 11 Reporting the Activity Date of This ADC | [46] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 52.50% (Day 27) | High Mesothelin expression (MSLN+++; IHC 3+) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive xenograft tumor models. Mice were subcutaneously inoculated with 5x106 MSLN positive T1889 cells mixed with Matrigel. Treatment then began, and the experiments ran for a total of 4 weeks. The first treatment cohort of 32 mice consisted of vehicle control, isotype ADC control, and 15 mg/kg ARav, dosed at Q7D via intraperitoneal injection. A second treatment cohort of 30 mice occurred with groups consisting of vehicle control (Q14D), 7.5 mg/kg ARav (Q14D) monotherapy, 7.5 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy. A third treatment cohort of 32 mice occurred with groups consisting of vehicle control (Q14D), 3.75 mg/kg ARav (Q14D) monotherapy, 3.75 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing uterine carcinosarcoma PDX model (PDX: T1889) | ||||
| Experiment 12 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
54.00%
|
Positive Mesothelin expression (MSLN+++/++) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing high-grade serous ovarian cancer PDX model (PDX: OVCAR-8) | ||||
| Experiment 13 Reporting the Activity Date of This ADC | [45] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
57.10% (Day 70)
|
High Mesothelin expression (MSLN+++; IHC 3+) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive xenograft tumor models. Mice were subcutaneously inoculated with 5x106 MSLN positive T1889 cells mixed with Matrigel. Treatment then began, and the experiments ran for a total of 4 weeks. The first treatment cohort of 32 mice consisted of vehicle control, isotype ADC control, and 15 mg/kg ARav, dosed at Q7D via intraperitoneal injection. A second treatment cohort of 30 mice occurred with groups consisting of vehicle control (Q14D), 7.5 mg/kg ARav (Q14D) monotherapy, 7.5 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy. A third treatment cohort of 32 mice occurred with groups consisting of vehicle control (Q14D), 3.75 mg/kg ARav (Q14D) monotherapy, 3.75 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing uterine carcinosarcoma PDX model (PDX: Cx-03) | ||||
| Experiment 14 Reporting the Activity Date of This ADC | [46] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 63.10% (Day 27) | High Mesothelin expression (MSLN+++; IHC 3+) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive xenograft tumor models. Mice were subcutaneously inoculated with 5x106 MSLN positive T1889 cells mixed with Matrigel. Treatment then began, and the experiments ran for a total of 4 weeks. The first treatment cohort of 32 mice consisted of vehicle control, isotype ADC control, and 15 mg/kg ARav, dosed at Q7D via intraperitoneal injection. A second treatment cohort of 30 mice occurred with groups consisting of vehicle control (Q14D), 7.5 mg/kg ARav (Q14D) monotherapy, 7.5 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy. A third treatment cohort of 32 mice occurred with groups consisting of vehicle control (Q14D), 3.75 mg/kg ARav (Q14D) monotherapy, 3.75 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing uterine carcinosarcoma PDX model (PDX: T1889) | ||||
| Experiment 15 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
64.00%
|
Moderate MSLN expression (MSLN++) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing high-grade serous papillary carcinoma PDX model (PDX: ST409) | ||||
| Experiment 16 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
64.00%
|
Positive Mesothelin expression (MSLN+++/++) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing ovarian cancer PDX model (PDX: Ov6668) | ||||
| Experiment 17 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 64.30% (Day 21) | Low Mesothelin expression (MSLN+; IHC 1+) | ||
| Method Description |
For subcutaneous tumor models,female NMRI nu/nu mice (18-25 g,7-10 weeks) from Taconic M&B were implanted on day 0 with either 3 x106 MIA PaCa-2/vector,3 x106 MIA PaCa-2/meso,1 x106 HT-29/vector,1 x106 HT-29/meso,3 x106 OVCAR-3,or 3 x106 NCI-H226 cells suspended in 0.1 mL 50% Matrigel. Patient-derived pancreatic (PAXF736) model was performed at Oncotest GmbH and ovarian (OVCAR6719) and mesothelioma (Meso7212) models at EPO Berlin-Buch GmbH. 0.05 mg/kg , 0.2 mg/kg Anetumab ravtansine was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing colon carcinoma model PDX model (PDX: HT-29/meso) | ||||
| Experiment 18 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
65.00%
|
Positive Mesothelin expression (MSLN+++/++) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing high-grade serous ovarian cancer PDX model (PDX: OVCAR-8) | ||||
| Experiment 19 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 66.70% (Day 153) | Low Mesothelin expression (MSLN+; IHC 1+) | ||
| Method Description |
For subcutaneous tumor models,female NMRI nu/nu mice (18-25 g,7-10 weeks) from Taconic M&B were implanted on day 0 with either 3 x106 MIA PaCa-2/vector,3 x106 MIA PaCa-2/meso,1 x106 HT-29/vector,1 x106 HT-29/meso,3 x106 OVCAR-3,or 3 x106 NCI-H226 cells suspended in 0.1 mL 50% Matrigel. Patient-derived pancreatic (PAXF736) model was performed at Oncotest GmbH and ovarian (OVCAR6719) and mesothelioma (Meso7212) models at EPO Berlin-Buch GmbH. 0.03 mg/kg , 0.05 mg/kg , 0.2 mg/kg Anetumab ravtansine was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing mesothelioma PDX model (PDX: NCI-H226) | ||||
| Experiment 20 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
73.00%
|
Positive Mesothelin expression (MSLN+++/++) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing ovarian cancer PDX model (PDX: Ov6668) | ||||
| Experiment 21 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
74.00%
|
Positive Mesothelin expression (MSLN+++/++) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing high-grade serous ovarian cancer PDX model (PDX: ST081) | ||||
| Experiment 22 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
75.00%
|
Positive Mesothelin expression (MSLN+++/++) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing serous papillary carcinoma PDX model (PDX: ST467) | ||||
| Experiment 23 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
83.00%
|
Positive Mesothelin expression (MSLN+++/++) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing high-grade serous ovarian cancer PDX model (PDX: OVCAR-3) | ||||
| Experiment 24 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
83.00%
|
Negative Mesothelin expression (MSLN-) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing ovarian cancer PDX model (PDX: Ov6668) | ||||
| Experiment 25 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 83.30% (Day 90) | Low Mesothelin expression (MSLN+; IHC 1+) | ||
| Method Description |
For subcutaneous tumor models,female NMRI nu/nu mice (18-25 g,7-10 weeks) from Taconic M&B were implanted on day 0 with either 3x106 MIA PaCa-2/vector, 3x106 MIA PaCa-2/meso,1x106 HT-29/vector, 1x106 HT-29/meso, 3x106 OVCAR-3, or 3x106 NCI-H226 cells suspended in 0.1 mL 50% Matrigel. Patient-derived pancreatic (PAXF736) model was performed at Oncotest GmbH and ovarian (OVCAR6719) and mesothelioma (Meso7212) models at EPO Berlin-Buch GmbH. BAY 94-9343 administered at 0.05 mg/kg, 0.2 mg/kg (corresponding to 14.3 mg/kg ADC; Q3Dx3).
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian cancer PDX model (PDX: OVCAR6719) | ||||
| Experiment 26 Reporting the Activity Date of This ADC | [46] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 83.90% (Day 27) | Low Mesothelin expression (MSLN+; IHC 1+) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive xenograft tumor models. Mice were subcutaneously inoculated with 5x106 MSLN positive T1889 cells mixed with Matrigel. Treatment then began, and the experiments ran for a total of 4 weeks. The first treatment cohort of 32 mice consisted of vehicle control, isotype ADC control, and 15 mg/kg ARav, dosed at Q7D via intraperitoneal injection. A second treatment cohort of 30 mice occurred with groups consisting of vehicle control (Q14D), 7.5 mg/kg ARav (Q14D) monotherapy, 7.5 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy. A third treatment cohort of 32 mice occurred with groups consisting of vehicle control (Q14D), 3.75 mg/kg ARav (Q14D) monotherapy, 3.75 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing uterine carcinosarcoma PDX model (PDX: T1889) | ||||
| Experiment 27 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
85.00%
|
Negative Mesothelin expression (MSLN-) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing high-grade serous ovarian cancer PDX model (PDX: ST081) | ||||
| Experiment 28 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.00% (Day 60) | Low Mesothelin expression (MSLN+; IHC 1+) | ||
| Method Description |
For subcutaneous tumor models,female NMRI nu/nu mice (18-25 g,7-10 weeks) from Taconic M&B were implanted on day 0 with either 3x106 MIA PaCa-2/vector, 3x106 MIA PaCa-2/meso,1x106 HT-29/vector, 1x106 HT-29/meso, 3x106 OVCAR-3, or 3x106 NCI-H226 cells suspended in 0.1 mL 50% Matrigel. Patient-derived pancreatic (PAXF736) model was performed at Oncotest GmbH and ovarian (OVCAR6719) and mesothelioma (Meso7212) models at EPO Berlin-Buch GmbH. BAY 94-9343 administered at 0.05 mg/kg, 0.2 mg/kg (corresponding to 14.3 mg/kg ADC; Q3Dx3).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing pancreatic tumor PDX model (PDX: PAXF736) | ||||
| Experiment 29 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
90.00%
|
Positive Mesothelin expression (MSLN+++/++) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing ovarian cancer PDX model (PDX: OVCAR-3) | ||||
| Experiment 30 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.40% (Day 61) | Low MSLN expresion (MSLN+; 900 MSLN molecules/cell) | ||
| Method Description |
For subcutaneous tumor models,female NMRI nu/nu mice (18-25 g,7-10 weeks) from Taconic M&B were implanted on day 0 with either 3 x106 MIA PaCa-2/vector,3 x106 MIA PaCa-2/meso,1 x106 HT-29/vector,1 x106 HT-29/meso,3 x106 OVCAR-3,or 3 x106 NCI-H226 cells suspended in 0.1 mL 50% Matrigel. Patient-derived pancreatic (PAXF736) model was performed at Oncotest GmbH and ovarian (OVCAR6719) and mesothelioma (Meso7212) models at EPO Berlin-Buch GmbH. 0.05 mg/kg , 0.2 mg/kg Anetumab ravtansine was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing ovarian cancer PDX model (PDX: OVCAR-36) | ||||
| Experiment 31 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.60% (Day 153) | High MSLN expresion (MSLN+++; 41,887 MSLN molecules/cell) | ||
| Method Description |
For subcutaneous tumor models,female NMRI nu/nu mice (18-25 g,7-10 weeks) from Taconic M&B were implanted on day 0 with either 3 x106 MIA PaCa-2/vector,3 x106 MIA PaCa-2/meso,1 x106 HT-29/vector,1 x106 HT-29/meso,3 x106 OVCAR-3,or 3 x106 NCI-H226 cells suspended in 0.1 mL 50% Matrigel. Patient-derived pancreatic (PAXF736) model was performed at Oncotest GmbH and ovarian (OVCAR6719) and mesothelioma (Meso7212) models at EPO Berlin-Buch GmbH. 0.03 mg/kg , 0.05 mg/kg , 0.2 mg/kg Anetumab ravtansine was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing mesothelioma PDX model (PDX: NCI-H226) | ||||
| Experiment 32 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.20% (Day 61) | Moderate MSLN expresion (MSLN++; 1,260 MSLN molecules/cell) | ||
| Method Description |
For subcutaneous tumor models,female NMRI nu/nu mice (18-25 g,7-10 weeks) from Taconic M&B were implanted on day 0 with either 3 x106 MIA PaCa-2/vector,3 x106 MIA PaCa-2/meso,1 x106 HT-29/vector,1 x106 HT-29/meso,3 x106 OVCAR-3,or 3 x106 NCI-H226 cells suspended in 0.1 mL 50% Matrigel. Patient-derived pancreatic (PAXF736) model was performed at Oncotest GmbH and ovarian (OVCAR6719) and mesothelioma (Meso7212) models at EPO Berlin-Buch GmbH. 0.01 mg/kg , 0.05 mg/kg , 0.2 mg/kg Anetumab ravtansine was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing ovarian cancer PDX model (PDX: OVCAR-36) | ||||
| Experiment 33 Reporting the Activity Date of This ADC | [46] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.70% (Day 27) | High MSLN expresion (MSLN+++; 53,497 MSLN molecules/cell) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive xenograft tumor models. Mice were subcutaneously inoculated with 5x106 MSLN positive T1889 cells mixed with Matrigel. Treatment then began, and the experiments ran for a total of 4 weeks. The first treatment cohort of 32 mice consisted of vehicle control, isotype ADC control, and 15 mg/kg ARav, dosed at Q7D via intraperitoneal injection. A second treatment cohort of 30 mice occurred with groups consisting of vehicle control (Q14D), 7.5 mg/kg ARav (Q14D) monotherapy, 7.5 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy. A third treatment cohort of 32 mice occurred with groups consisting of vehicle control (Q14D), 3.75 mg/kg ARav (Q14D) monotherapy, 3.75 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing uterine carcinosarcoma PDX model (PDX: T1889) | ||||
| Experiment 34 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
96.00%
|
Moderate Mesothelin expression (MSLN++; IHC 2+) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing high-grade serous papillary carcinoma PDX model (PDX: ST270) | ||||
| Experiment 35 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
96.00%
|
Moderate Mesothelin expression (MSLN++; IHC 2+) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing ovarian cancer PDX model (PDX: OVCAR-3) | ||||
| Experiment 36 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 20) | Moderate Mesothelin expression (MSLN++; IHC 2+) | ||
| Method Description |
For subcutaneous tumor models,female NMRI nu/nu mice (18-25 g, 7-10 weeks) from Taconic M&B were implanted on day 0 with either 3 x106 MIA PaCa-2/vector,3 x106 MIA PaCa-2/meso,1 x106 HT-29/vector,1 x106 HT-29/meso,3 x106 OVCAR-3,or 3 x106 NCI-H226 cells suspended in 0.1 mL 50% Matrigel. Patient-derived pancreatic (PAXF736) model was performed at Oncotest GmbH and ovarian (OVCAR6719) and mesothelioma (Meso7212) models at EPO Berlin-Buch GmbH. 0.01 mg/kg , 0.05 mg/kg , 0.2 mg/kg Anetumab ravtansine was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing pancreatic carcinoma PDX model (PDX: MIA PaCa-2/meso) | ||||
| Experiment 37 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 20) | Moderate Mesothelin expression (MSLN++; IHC 2+) | ||
| Method Description |
For subcutaneous tumor models,female NMRI nu/nu mice (18-25 g, 7-10 weeks) from Taconic M&B were implanted on day 0 with either 3 x106 MIA PaCa-2/vector,3 x106 MIA PaCa-2/meso,1 x106 HT-29/vector,1 x106 HT-29/meso,3 x106 OVCAR-3,or 3 x106 NCI-H226 cells suspended in 0.1 mL 50% Matrigel. Patient-derived pancreatic (PAXF736) model was performed at Oncotest GmbH and ovarian (OVCAR6719) and mesothelioma (Meso7212) models at EPO Berlin-Buch GmbH. 0.01 mg/kg , 0.05 mg/kg , 0.2 mg/kg Anetumab ravtansine was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing pancreatic carcinoma PDX model (PDX: MIA PaCa-2/meso) | ||||
| Experiment 38 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 21) | High Mesothelin expression (MSLN+++; IHC 3+) | ||
| Method Description |
For subcutaneous tumor models,female NMRI nu/nu mice (18-25 g, 7-10 weeks) from Taconic M&B were implanted on day 0 with either 3 x106 MIA PaCa-2/vector,3 x106 MIA PaCa-2/meso,1 x106 HT-29/vector,1 x106 HT-29/meso,3 x106 OVCAR-3,or 3 x106 NCI-H226 cells suspended in 0.1 mL 50% Matrigel. Patient-derived pancreatic (PAXF736) model was performed at Oncotest GmbH and ovarian (OVCAR6719) and mesothelioma (Meso7212) models at EPO Berlin-Buch GmbH. 0.01 mg/kg , 0.05 mg/kg , 0.2 mg/kg Anetumab ravtansine was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing colon carcinoma model PDX model (PDX: HT-29/meso) | ||||
| Experiment 39 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 90) | Moderate Mesothelin expression (MSLN++; IHC 2+) | ||
| Method Description |
For subcutaneous tumor models,female NMRI nu/nu mice (18-25 g, 7-10 weeks) from Taconic M&B were implanted on day 0 with either 3 x106 MIA PaCa-2/vector,3 x106 MIA PaCa-2/meso,1 x106 HT-29/vector,1 x106 HT-29/meso,3 x106 OVCAR-3,or 3 x106 NCI-H226 cells suspended in 0.1 mL 50% Matrigel. Patient-derived pancreatic (PAXF736) model was performed at Oncotest GmbH and ovarian (OVCAR6719) and mesothelioma (Meso7212) models at EPO Berlin-Buch GmbH. BAY 94-9343 administered at 0.05 mg/kg,0.2 mg/kg (corresponding to 14.3 mg/kg ADC; Q3Dx3).
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian cancer PDX model (PDX: OVCAR6719) | ||||
| Experiment 40 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 90) | High Mesothelin expression (MSLN+++; IHC 3+) | ||
| Method Description |
For subcutaneous tumor models,female NMRI nu/nu mice (18-25 g, 7-10 weeks) from Taconic M&B were implanted on day 0 with either 3 x106 MIA PaCa-2/vector,3 x106 MIA PaCa-2/meso,1 x106 HT-29/vector,1 x106 HT-29/meso,3 x106 OVCAR-3,or 3 x106 NCI-H226 cells suspended in 0.1 mL 50% Matrigel. Patient-derived pancreatic (PAXF736) model was performed at Oncotest GmbH and ovarian (OVCAR6719) and mesothelioma (Meso7212) models at EPO Berlin-Buch GmbH. BAY 94-9343 administered at 0.05 mg/kg,0.2 mg/kg (corresponding to 14.3 mg/kg ADC; Q3Dx3).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing mesothelioma model PDX model (PDX: Meso7212) | ||||
| Experiment 41 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 90) | High Mesothelin expression (MSLN+++; IHC 3+) | ||
| Method Description |
For subcutaneous tumor models,female NMRI nu/nu mice (18-25 g, 7-10 weeks) from Taconic M&B were implanted on day 0 with either 3 x106 MIA PaCa-2/vector,3 x106 MIA PaCa-2/meso,1 x106 HT-29/vector,1 x106 HT-29/meso,3 x106 OVCAR-3,or 3 x106 NCI-H226 cells suspended in 0.1 mL 50% Matrigel. Patient-derived pancreatic (PAXF736) model was performed at Oncotest GmbH and ovarian (OVCAR6719) and mesothelioma (Meso7212) models at EPO Berlin-Buch GmbH. BAY 94-9343 administered at 0.05 mg/kg,0.2 mg/kg (corresponding to 14.3 mg/kg ADC; Q3Dx3).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing mesothelioma model PDX model (PDX: Meso7212) | ||||
| Experiment 42 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00%
|
Moderate Mesothelin expression (MSLN++; IHC 2+) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing high-grade serous ovarian cancer PDX model (PDX: ST081) | ||||
| Experiment 43 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00%
|
High Mesothelin expression (MSLN+++; IHC 3+) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive ovarian cancer cell line- and patient-derived xenograft models. For the OVCAR-3 and OVCAR-8 xenograft models,tumor cells in Matrigel were inoculated subcutaneously to the right lower flank region of female nude/nude mice. In the monotherapy experiments,anetumab ravtansine was administered intravenously (i.v.) at 2.5 mg/kg three times every third day (Q3Dx3). For the in vivo combination studies with pegylated liposomal doxorubicin (PLD) or copanlisib in OVCAR-8 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,7,28,32 and 35,or on days 1,4,28 and 35,respectively. For the in vivo combination studies in OVCAR-3 xenografts,anetumab ravtansine was administered i.v. at 2.5 mg/kg on days 1,4,43 and 46. For the Ov6668 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg on day 1 and at 15 mg/kg on days 16,30,43,57 and 71. For the ST081 xenografts,anetumab ravtansine was administered i.v. at 3.75 mg/kg every second week (Q2W). PLD was administered i.v. at 4 mg/kg on days 1,7,28 and 35 (OVCAR-8 xenografts),on days 1 and 30 (Ov6668 xenografts) or on days 0 and 7 (ST081 xenografts). Carboplatin was administered i.v. at 80 mg/kg QWx2. Copanlisib was administered at 10 mg/kg,2 days on/5 days off,i.v.,starting on day 2. Bevacizumab was administered intraperitoneally (i.p.) at 0.3 mg/kg,every fifth day (Q5D).
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing high-grade ovarian cancer PDX model (PDX: ST103) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [49] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
25.00% (Day 70)
|
High MSLN expression (MSLN+++) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive xenograft tumor models. Four groups of mice,each consisting of 58 animals was set up and vital Hela cells were inoculated in a dose of 110e5 cells in 100 l by subcutaneous injection in the left inner flank on day 0. Animals in the treatment groups received 2 mg/kg,5 mg/kg,and 10 mg/kg anetumab ravtansine in 200 l injection buffer twice weekly by i.p. injection. Animals in the control group received injection buffer only.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing hela CDX model | ||||
| In Vitro Model | Endocervical adenocarcinoma | HeLa cells | CVCL_0030 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [45] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
25.00% (Day 70)
|
High MSLN expression (MSLN+++) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive xenograft tumor models. Mice were subcutaneously inoculated with 5x106 MSLN positive T1889 cells mixed with Matrigel. Treatment then began, and the experiments ran for a total of 4 weeks. The first treatment cohort of 32 mice consisted of vehicle control, isotype ADC control, and 15 mg/kg ARav, dosed at Q7D via intraperitoneal injection. A second treatment cohort of 30 mice occurred with groups consisting of vehicle control (Q14D), 7.5 mg/kg ARav (Q14D) monotherapy, 7.5 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy. A third treatment cohort of 32 mice occurred with groups consisting of vehicle control (Q14D), 3.75 mg/kg ARav (Q14D) monotherapy, 3.75 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing cervical squamous cell carcinoma hela CDX model | ||||
| In Vitro Model | Endocervical adenocarcinoma | HeLa cells | CVCL_0030 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [45] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
50.00% (Day 70)
|
High MSLN expression (MSLN+++) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive xenograft tumor models. Mice were subcutaneously inoculated with 5x106 MSLN positive T1889 cells mixed with Matrigel. Treatment then began, and the experiments ran for a total of 4 weeks. The first treatment cohort of 32 mice consisted of vehicle control, isotype ADC control, and 15 mg/kg ARav, dosed at Q7D via intraperitoneal injection. A second treatment cohort of 30 mice occurred with groups consisting of vehicle control (Q14D), 7.5 mg/kg ARav (Q14D) monotherapy, 7.5 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy. A third treatment cohort of 32 mice occurred with groups consisting of vehicle control (Q14D), 3.75 mg/kg ARav (Q14D) monotherapy, 3.75 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing cervical squamous cell carcinoma hela CDX model | ||||
| In Vitro Model | Endocervical adenocarcinoma | HeLa cells | CVCL_0030 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [49] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
75.00% (Day 70)
|
Negative MSLN expression (MSLN-) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive xenograft tumor models. Four groups of mice,each consisting of 58 animals was set up and vital Hela cells were inoculated in a dose of 110e5 cells in 100 l by subcutaneous injection in the left inner flank on day 0. Animals in the treatment groups received 2 mg/kg,5 mg/kg,and 10 mg/kg anetumab ravtansine in 200 l injection buffer twice weekly by i.p. injection. Animals in the control group received injection buffer only.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing hela CDX model | ||||
| In Vitro Model | Endocervical adenocarcinoma | HeLa cells | CVCL_0030 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [45] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
85.70% (Day 70)
|
Moderate MSLN expression (MSLN++) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive xenograft tumor models. Mice were subcutaneously inoculated with 5x106 MSLN positive T1889 cells mixed with Matrigel. Treatment then began, and the experiments ran for a total of 4 weeks. The first treatment cohort of 32 mice consisted of vehicle control, isotype ADC control, and 15 mg/kg ARav, dosed at Q7D via intraperitoneal injection. A second treatment cohort of 30 mice occurred with groups consisting of vehicle control (Q14D), 7.5 mg/kg ARav (Q14D) monotherapy, 7.5 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy. A third treatment cohort of 32 mice occurred with groups consisting of vehicle control (Q14D), 3.75 mg/kg ARav (Q14D) monotherapy, 3.75 mg/kg ARav (Q14D) and 4 mg/kg cisplatin (Q7D) combination therapy, and 4 mg/kg cisplatin (Q7D) monotherapy.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing cervical squamous cell carcinoma hela CDX model | ||||
| In Vitro Model | Endocervical adenocarcinoma | HeLa cells | CVCL_0030 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [49] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
88.00% (Day 70)
|
Positive Mesothelin expression (MSLN+++/++) | ||
| Method Description |
The in vivo antitumor activity of Anetumab ravtansine was evaluated in a Mesothelin positive xenograft tumor models. Four groups of mice,each consisting of 58 animals was set up and vital Hela cells were inoculated in a dose of 110e5 cells in 100 l by subcutaneous injection in the left inner flank on day 0. Animals in the treatment groups received 2 mg/kg,5 mg/kg,and 10 mg/kg anetumab ravtansine in 200 l injection buffer twice weekly by i.p. injection. Animals in the control group received injection buffer only.
Click to Show/Hide
|
||||
| In Vivo Model | Mesothelin-expressing hela CDX model | ||||
| In Vitro Model | Endocervical adenocarcinoma | HeLa cells | CVCL_0030 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00 nM
|
|||
| Method Description |
The inhibitory activity of BAY 94-9343 against cancer cell growth was evaluated in various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | MIA PaCa-2 cells | CVCL_0428 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.05 nM
|
|||
| Method Description |
The inhibitory activity of BAY 94-9343 against cancer cell growth was evaluated in various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Colon adenocarcinoma | HT-29 cells | CVCL_0320 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.59 nM
|
|||
| Method Description |
The inhibitory activity of BAY 94-9343 against cancer cell growth was evaluated in various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | MIA PaCa-2 cells (MSLN expression) | CVCL_0428 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.59 nM
|
|||
| Method Description |
The inhibitory activity of BAY 94-9343 against cancer cell growth was evaluated in various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.10 nM
|
|||
| Method Description |
The inhibitory activity of anetumab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.72 nM
|
|||
| Method Description |
The inhibitory activity of BAY 94-9343 against cancer cell growth was evaluated in various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Pleural epithelioid mesothelioma | NCI-H226 cells | CVCL_1544 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.90 nM
|
Low MSLN expresion (MSLN+; 952 MSLN molecules/cell) | ||
| Method Description |
The inhibitory activity of anetumab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Ovarian adenocarcinoma | BG1 cells | CVCL_6570 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
7.15 nM
|
|||
| Method Description |
The inhibitory activity of BAY 94-9343 against cancer cell growth was evaluated in various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Colon adenocarcinoma | HT-29 cells (MSLN expression) | CVCL_0320 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
10.90 nM
|
|||
| Method Description |
The inhibitory activity of anetumab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
11.90 nM
|
Moderate MSLN expresion (MSLN++; 3,875 MSLN molecules/cell) | ||
| Method Description |
The inhibitory activity of anetumab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-5 cells | CVCL_1628 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
15.40 nM
|
|||
| Method Description |
The inhibitory activity of anetumab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
20.70 nM
|
|||
| Method Description |
The inhibitory activity of anetumab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | A2780 cells | CVCL_0134 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
20.80 nM
|
|||
| Method Description |
The inhibitory activity of anetumab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | EFO-21 cells | CVCL_0029 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
32.50 nM
|
Moderate MSLN expresion (MSLN++; 9,648 MSLN molecules/cell) | ||
| Method Description |
The inhibitory activity of anetumab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro.
|
||||
| In Vitro Model | High grade ovarian serous adenocarcinoma | OVCAR-8 cells | CVCL_1629 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
41.90 nM
|
|||
| Method Description |
The inhibitory activity of anetumab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
42.40 nM
|
|||
| Method Description |
The inhibitory activity of anetumab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro.
|
||||
| In Vitro Model | High grade ovarian serous adenocarcinoma | NCI-ADR-RES cells | CVCL_1452 | ||
Cantuzumab ravtansine [Phase 2 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [26] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT00620607 | Phase Status | Phase 2 | ||
| Clinical Description |
A phase 2, open label, multiple center study of HUC242-DM4 given as an intravenous infusion once every three weeks to patients with metastatic gastric or gastroesophageal junction carcinomas.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [35] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT00352131 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 study to assess the safety and pharmacokinetics of huC242-DM4 administered as a single intravenous infusion once every three weeks to subjects with solid tumors.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [36] | ||||
| Patients Enrolled |
Metastatic or inoperable colorectal, pancreatic, and other CanAg-expressing tumors who have failed standard therapy (about 95% of pts. had received = 4 prior chemotherapy regimens).
|
||||
| Administration Dosage |
A single intravenous (IV) infusion once every three weeks, 18, 36, 60, 90, 126, 168, 223, and 297 mg/m2.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT00352131 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 study to assess the safety and pharmacokinetics of huC242-DM4 administered as a single intravenous infusion once every three weeks to subjects with solid tumors.
|
||||
| Primary Endpoint |
HuC242-DM4 was well tolerated at the 168 mg/m2 dose level. The MTD is not yet defined.
|
||||
| Other Endpoint |
No clinically significant myelosuppression and no formation of antibody to humanized antibody (HAHA) or drug (HADA).
|
||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 48.85% (Day 20) | Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
MOLP-8 cells (1.5x107 cells per mouse) suspended in a 50:50 mixture of serum free media and matrigel were injected subcutaneously in the area under the right shoulder in 100 ul. Nine groups (n=6) were treated with a single intravenous injection of ADCs, each at doses of 250 ug/kg.
|
||||
| In Vivo Model | MOLP-8 CDX model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
SAR-566658 [Phase 2 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [33] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02984683 | Phase Status | Phase 1 | ||
| Clinical Description |
Open-label phase 2 study evaluating efficacy and safety of SAR566658 treatment in patients with CA6 positive metastatic triple negative breast cancer.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [34] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT01156870 | Phase Status | Phase 1 | ||
| Clinical Description |
Dose escalation, safety and pharmacokinetic, first in man study, of SAR566658 administered as a single agent by intravenous infusion in adult patients with CA6-positive and refractory solid tumors.
|
||||
Indatuximab ravtansine [Phase 1/2 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
3.20% (on the single-dose regimen)
5.90% (the multi-dose regimen) |
|||
| Patients Enrolled |
Relapsed and/or refractory multiple myeloma (MM) previously treated with an immunomodulatory drug and a proteasome inhibitor.
|
||||
| Administration Dosage |
In the first-in-human study, indatuximab ravtansine (10, 20, 40, 80, 120, 160, 200 mg/m2) was administered to 32 patients on day 1 of each 21-day cycle. The MTD was 160 mg/m2. In the phase I/IIa study, indatuximab ravtansine (40, 50, 65, 80, 100, 120, 140, 160 mg/m2) was administered to 35 patients on days 1, 8, and 15 of each 28-day cycle, and the MTD/recommended phase II dose was 140 mg/m2.
Click to Show/Hide
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT00723359 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 dose escalation study to evaluate maximum tolerated dose (MTD), pharmacokinetics (PK), and safety of BT062 in subjects with relapsed or relapsed/refractory multiple myeloma.
|
||||
| Primary Endpoint |
The 160 mg/m2 dose was therefore defined as MTD for the Single-dose Regimen.
|
||||
| Other Endpoint |
Most (88.00%) adverse events were grade 1 or 2, the most common being diarrhea and fatigue. There was rapid clearance of indatuximab ravtansine and no relevant accumulation.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [52] | ||||
| Patients Enrolled |
Relapsed or refractory multiple myeloma, and ECOG performance status or Zubrod score of 2 or below.
|
||||
| Administration Dosage |
Intravenously on days 1, 8, and 15 of each 28-day cycle in escalating dose levels of 80 mg/m2, 100 mg/m2, and 120 mg/m2, with lenalidomide (25 mg; days 1 to 21 every 28 days orally) and dexamethasone (20-40 mg; days 1, 8, 15, and 22 every 28 days) (phase 1).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01638936 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1/2a multi-dose escalation study of BT062 in combination with lenalidomide or pomalidomide and dexamethasone in subjects with relapsed or relapsed/refractory multiple myeloma.
|
||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 39) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 1 mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF1384) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 2 mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF1384) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 39) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 4 mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF1384) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 25.00% (Day 38) | High CD138 expression (CD138+++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 1 mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF401) | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 55.00% (Day 38) | High CD138 expression (CD138+++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 2 mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF401) | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 55.00% (Day 39) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine combination docetaxel against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated docetaxel with 10 mg/kg and treated indatuximab ravtansine with 2 mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF1384) | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 70.00% (Day 39) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 8mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF1384) | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.50% (Day 38) | High CD138 expression (CD138+++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 4 mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF401) | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.80% (Day 38) | High CD138 expression (CD138+++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 8mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF401) | ||||
| Experiment 10 Reporting the Activity Date of This ADC | [53] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 38) | High CD138 expression (CD138+++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine combination docetaxel against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated docetaxel with 10 mg/kg and treated indatuximab ravtansine with 2 mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF401) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 38.86% (Day 14) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine combination lenalidomide against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 5.3 mg/kg body weight for 14 days.
|
||||
| In Vivo Model | Multiple myeloma CDX model | ||||
| In Vitro Model | Multiple myeloma | Multiple myeloma cells | Homo sapiens | ||
| Experiment 2 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 40.42% (Day 17) | High CD138 expression (CD138+++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 2 mg/kg/day.
|
||||
| In Vitro Model | Plasma cell myeloma | MMXF L363 cells | Homo sapiens | ||
| Experiment 3 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 50.00% (Day 14) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 5.3 mg/kg body weight for 14 days.
|
||||
| In Vivo Model | Multiple myeloma CDX model | ||||
| In Vitro Model | Multiple myeloma | Multiple myeloma cells | Homo sapiens | ||
| Experiment 4 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 55.69% (Day 14) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine combination lenalidomide against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 10.6 mg/kg body weight for 14 days.
|
||||
| In Vivo Model | Multiple myeloma CDX model | ||||
| In Vitro Model | Multiple myeloma | Multiple myeloma cells | Homo sapiens | ||
| Experiment 5 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 59.90% (Day 14) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 10.6 mg/kg body weight for 14 days.
|
||||
| In Vivo Model | Multiple myeloma CDX model | ||||
| In Vitro Model | Multiple myeloma | Multiple myeloma cells | Homo sapiens | ||
| Experiment 6 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 65.34% (Day 14) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 21.2 mg/kg body weight for 14 days.
|
||||
| In Vivo Model | Multiple myeloma CDX model | ||||
| In Vitro Model | Multiple myeloma | Multiple myeloma cells | Homo sapiens | ||
| Experiment 7 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.26% (Day 17) | High CD138 expression (CD138+++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 4 mg/kg/day.
|
||||
| In Vitro Model | Plasma cell myeloma | MMXF L363 cells | Homo sapiens | ||
| Experiment 8 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.27% (Day 17) | High CD138 expression (CD138+++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine+lenalidomide (Len; 20 mg/kg/day) and dexamethasone (1.25 mg/kg/day) against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated IR with 2 mg/kg/day.
|
||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 79.20% (Day 14) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine combination lenalidomide against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 21.2 mg/kg body weight for 14 days.
|
||||
| In Vivo Model | Multiple myeloma CDX model | ||||
| In Vitro Model | Multiple myeloma | Multiple myeloma cells | Homo sapiens | ||
| Experiment 10 Reporting the Activity Date of This ADC | [54] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 92.05% (Day 17) | High CD138 expression (CD138+++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine+lenalidomide (Len; 20 mg/kg/day) and dexamethasone (1.25 mg/kg/day) against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated IR with 4 mg/kg/day.
|
||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
MEN-1309 [Phase 1]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [55] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04064359 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1, open-label, dose finding study to assess the safety, tolerability, PK, and preliminary efficacy of OBT076, a CD205-directed ADC, in recurrent and/or metastatic CD205+ solid tumors.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [55] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03403725 | Phase Status | Phase 1 | ||
| Clinical Description |
Open-label, multicenter, phase 1 dose escalation study of MEN1309, a CD205 antibody-drug conjugate,in patients with CD205-positive metastatic solid tumors and non-Hodgkin lymphoma.
|
||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 42) | High CD205 expression (CD205+++; IHC 3+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,1.25 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Pancreas cancer PDX model (PDX: AspC1) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 11.20% (Day 42) | High CD205 expression (CD205+++; IHC 3+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,2.5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Pancreas cancer PDX model (PDX: AspC1) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 14.50% (Day 42) | Negative CD205 expression (CD205-; IHC 0) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,3.5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Pancreas cancer PDX model (PDX: AspC1) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 16.50% (Day 42) | Negative CD205 expression (CD205-; IHC 0) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Pancreas cancer PDX model (PDX: AspC1) | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 20.50% (Day 33) | Negative CD205 expression (CD205-; IHC 0) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,1.25 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Pancreas cancer PDX model (PDX: HPAFII) | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 37.80% (Day 50) | Negative CD205 expression (CD205-; IHC 0) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: PDX-22) | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 38.70% (Day 18) | High CD205 expression (CD205+++; IHC 3+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Bladder cancer PDX model (PDX: PDX-CTG-1652) | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 49.30% (Day 18) | Low CD205 expression (CD205+; IHC 1+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Bladder cancer PDX model (PDX: PDX-CTG-1388) | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 50.00% (Day 57) | Moderate CD205 expression (CD205++; IHC 2+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Pancreas cancer PDX model (PDX: PDX-21) | ||||
| Experiment 10 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 60.20% (Day 33) | Moderate CD205 expression (CD205++; IHC 2+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,2.5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Pancreas cancer PDX model (PDX: HPAFII) | ||||
| Experiment 11 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 70.60% (Day 110) | Low CD205 expression (CD205+; IHC 1+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: PDX-P6P) | ||||
| Experiment 12 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.60% (Day 33) | High CD205 expression (CD205+++; IHC 3+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Pancreas cancer PDX model (PDX: HPAFII) | ||||
| Experiment 13 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.40% (Day 56) | High CD205 expression (CD205+++; IHC 3+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: PDX-347) | ||||
| Experiment 14 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.90% (Day 33) | High CD205 expression (CD205+++; IHC 3+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,10 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Pancreas cancer PDX model (PDX: HPAFII) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 22.70% (Day 50) | Moderate CD205 expression (CD205++; IHC 2+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,1.25 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer HCC70 CDX model | ||||
| In Vitro Model | Breast ductal carcinoma | HCC70 cells | CVCL_1270 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.60% (Day 40) | Moderate CD205 expression (CD205++) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,1.25 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Urinary bladder cancer SW780 model | ||||
| In Vitro Model | Bladder carcinoma | SW780 cells | CVCL_1728 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 63.70% (Day 40) | Low CD205 expression (CD205+; IHC 1+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,1.25 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer HCC1806 CDX model | ||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 72.30% (Day 40) | High CD205 expression (CD205+++) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,2.5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Urinary bladder cancer SW780 model | ||||
| In Vitro Model | Bladder carcinoma | SW780 cells | CVCL_1728 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 74.00% (Day 25) | Low CD205 expression (CD205+; IHC 1+) | ||
| Method Description |
The antitumor activity of the ADCs was then evaluated in solid and hematologic mouse xenograft models. A total of 5x106-2x107 cells with or without Matrigel, were injected subcutaneously into the flanks of mice. The treatments were started when average tumor volume reached 88-300 mm3. Animals were randomly assigned into groups (6 mice/group), and treated with MEN1309/OBT076 intravenously with a single dose, 5 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Pancreas cancer CDX model | ||||
| In Vitro Model | Pancreatic cancer | Pancreatic cancer cells | Homo sapiens | ||
| Experiment 6 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 79.90% (Day 40) | Low CD205 expression (CD205+; IHC 1+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,2.5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer HCC1806 CDX model | ||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.00% (Day 40) | Low CD205 expression (CD205+; IHC 1+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,3.5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer HCC1806 CDX model | ||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.50% (Day 40) | Moderate CD205 expression (CD205++; IHC 2+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Urinary bladder cancer SW780 model | ||||
| In Vitro Model | Bladder carcinoma | SW780 cells | CVCL_1728 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.80% (Day 50) | Moderate CD205 expression (CD205++; IHC 2+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,3.5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer HCC70 CDX model | ||||
| In Vitro Model | Breast ductal carcinoma | HCC70 cells | CVCL_1270 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.20% (Day 50) | Low CD205 expression (CD205+; IHC 1+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,2.5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer HCC70 CDX model | ||||
| In Vitro Model | Breast ductal carcinoma | HCC70 cells | CVCL_1270 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.50% (Day 25) | Low CD205 expression (CD205+; IHC 1+) | ||
| Method Description |
The antitumor activity of the ADCs was then evaluated in solid and hematologic mouse xenograft models. A total of 5x106-2x107 cells with or without Matrigel, were injected subcutaneously into the flanks of mice. The treatments were started when average tumor volume reached 88-300 mm3. Animals were randomly assigned into groups (6 mice/group), and treated with MEN1309/OBT076 intravenously once weekly for 2 consecutive weeks, 5 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Diffuse Large B-Cell Lymphoma CDX model | ||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.60% (Day 40) | Low CD205 expression (CD205+; IHC 1+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer HCC1806 CDX model | ||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.70% (Day 50) | Low CD205 expression (CD205+; IHC 1+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,5 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer HCC70 CDX model | ||||
| In Vitro Model | Breast ductal carcinoma | HCC70 cells | CVCL_1270 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.80% (Day 40) | Moderate CD205 expression (CD205++; IHC 2+) | ||
| Method Description |
To test the in vivo efficacy of MEN1309/OBT076,different xenograft and PDX models were selected on the basis of the IHC analysis for CD205 staining in various tumor types. Efficacy of MEN1309/OBT076,administered with a q21dx3,10 mg/kg schedule,was determined by assessing the inhibition of tumor growth at the nadir of tumor volume in treated versus control mice and assessing mRECIST criteria adapted to the mouse from human RECIST. Mice were euthanized when tumors reached 2, 000 mm 3 or when the study endpoint was reached.
Click to Show/Hide
|
||||
| In Vivo Model | Urinary bladder cancer SW780 model | ||||
| In Vitro Model | Bladder carcinoma | SW780 cells | CVCL_1728 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [57] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
100.00 pM
|
High CD205 expression (CD205+++; IHC 3+) | ||
| Method Description |
The inhibitory activity of MEN-1309 against cancer cell growth against 42 B-cell lymphoma cell lines in vitro. The cells were treated with MEN-1309 for 50 days.
|
||||
| In Vitro Model | Mantle cell lymphoma | Mantle cell lymphoma cells | Homo sapiens | ||
| Experiment 2 Reporting the Activity Date of This ADC | [57] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
110.00 pM
|
High CD205 expression (CD205+++; IHC 3+) | ||
| Method Description |
The inhibitory activity of MEN-1309 against cancer cell growth against 42 B-cell lymphoma cell lines in vitro. The cells were treated with MEN-1309 for 50 days.
|
||||
| In Vitro Model | Marginal zone lymphoma | Marginal zone lymphoma cells | Homo sapiens | ||
| Experiment 3 Reporting the Activity Date of This ADC | [57] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
200.00 pM
|
High CD205 expression (CD205+++; IHC 3+) | ||
| Method Description |
The inhibitory activity of MEN-1309 against cancer cell growth against 42 B-cell lymphoma cell lines in vitro. The cells were treated with MEN-1309 for 50 days.
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | Diffuse large B-cell lymphoma cells | Homo sapiens | ||
| Experiment 4 Reporting the Activity Date of This ADC | [57] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
300.00 pM
|
Moderate CD205 expression (CD205++; IHC 2+) | ||
| Method Description |
The inhibitory activity of MEN-1309 against cancer cell growth against 42 B-cell lymphoma cell lines in vitro. The cells were treated with MEN-1309 for 50 days.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [57] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
310.00 pM
|
Low CD205 expression (CD205+; IHC 1+) | ||
| Method Description |
The inhibitory activity of MEN-1309 against cancer cell growth against 42 B-cell lymphoma cell lines in vitro. The cells were treated with MEN-1309 for 50 days.
|
||||
| In Vitro Model | B-cell chronic lymphocytic leukemia | PCL12 cells | CVCL_2H32 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.10 nM
|
High CD205 expression (CD205+++; IHC 3+) | ||
| Method Description |
The expression of the CD205 antigen was evaluated in a panel of human pancreas,bladder,colon cancer,and TNBC cell lines. Tumor cells were incubated with MEN1309/OBT076 for 72 hours at 37°C.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.26 nM
|
Low CD205 expression (CD205+; IHC 1+) | ||
| Method Description |
The expression of the CD205 antigen was evaluated in a panel of human pancreas,bladder,colon cancer,and TNBC cell lines. Tumor cells were incubated with MEN1309/OBT076 for 72 hours at 37°C.
|
||||
| In Vitro Model | Bladder carcinoma | SW780 cells | CVCL_1728 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.37 nM
|
|||
| Method Description |
The expression of the CD205 antigen was evaluated in a panel of human pancreas,bladder,colon cancer,and TNBC cell lines. Tumor cells were incubated with MEN1309/OBT076 for 72 hours at 37°C.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.40 nM
|
Negative CD205 expression (CD205-; IHC 0) | ||
| Method Description |
The expression of the CD205 antigen was evaluated in a panel of human pancreas,bladder,colon cancer,and TNBC cell lines. Tumor cells were incubated with MEN1309/OBT076 for 72 hours at 37°C.
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 10 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.43 nM
|
Moderate CD205 expression (CD205++; IHC 2+) | ||
| Method Description |
The expression of the CD205 antigen was evaluated in a panel of human pancreas,bladder,colon cancer,and TNBC cell lines. Tumor cells were incubated with MEN1309/OBT076 for 72 hours at 37°C.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | BxPC-3 cells | CVCL_0186 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.71 nM
|
Moderate CD205 expression (CD205++; IHC 2+) | ||
| Method Description |
The expression of the CD205 antigen was evaluated in a panel of human pancreas,bladder,colon cancer,and TNBC cell lines. Tumor cells were incubated with MEN1309/OBT076 for 72 hours at 37°C.
|
||||
| In Vitro Model | Breast ductal carcinoma | HCC70 cells | CVCL_1270 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.81 nM
|
Negative CD205 expression (CD205-; IHC 0) | ||
| Method Description |
The expression of the CD205 antigen was evaluated in a panel of human pancreas,bladder,colon cancer,and TNBC cell lines. Tumor cells were incubated with MEN1309/OBT076 for 72 hours at 37°C.
|
||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.82 nM
|
|||
| Method Description |
The expression of the CD205 antigen was evaluated in a panel of human pancreas,bladder,colon cancer,and TNBC cell lines. Tumor cells were incubated with MEN1309/OBT076 for 72 hours at 37°C.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.32 nM
|
Low CD205 expression (CD205+; IHC 1+) | ||
| Method Description |
The expression of the CD205 antigen was evaluated in a panel of human pancreas,bladder,colon cancer,and TNBC cell lines. Tumor cells were incubated with MEN1309/OBT076 for 72 hours at 37°C.
|
||||
| In Vitro Model | Pancreatic adenocarcinoma | SU.86.86 cells | CVCL_3881 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
14.20 nM
|
|||
| Method Description |
The expression of the CD205 antigen was evaluated in a panel of human pancreas,bladder,colon cancer,and TNBC cell lines. Tumor cells were incubated with MEN1309/OBT076 for 72 hours at 37°C.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
22.66 nM
|
Negative CD205 expression (CD205-; IHC 0) | ||
| Method Description |
The expression of the CD205 antigen was evaluated in a panel of human pancreas,bladder,colon cancer,and TNBC cell lines. Tumor cells were incubated with MEN1309/OBT076 for 72 hours at 37°C.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | AsPC-1 cells | CVCL_0152 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [56] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
36.23 nM
|
High CD205 expression (CD205+++; IHC 3+) | ||
| Method Description |
The expression of the CD205 antigen was evaluated in a panel of human pancreas,bladder,colon cancer,and TNBC cell lines. Tumor cells were incubated with MEN1309/OBT076 for 72 hours at 37°C.
|
||||
| In Vitro Model | Recurrent bladder carcinoma | HT-1197 cells | CVCL_1291 | ||
AbGn-107 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [58] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
11.40% (all)
|
|||
| Patients Enrolled |
Patients with locally advanced or metastatic G, CRC, PDA, or BIL cancer, previously treated, ECOG PS 0-1, positive AG-7 expression was not required.
|
||||
| Administration Dosage |
AbGn-107 administered iv Q4 weeks (from 0.10-1.20 mg/kg) and Q2 weeks (from 0.80-1.00 mg/kg).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02908451 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 dose escalation study, with cohort expansion, to evaluate the safety, tolerability, pharmacokinetics, and preliminary efficacy of ABGN-107 therapy in patients with chemo-refractory locally advanced, recurrent, or metastatic gastric, colorectal, pancreatic or biliary cancer.
|
||||
SAR-428926 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [59] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02575781 | Phase Status | Phase 1 | ||
| Clinical Description |
A first-in-human phase 1 dose escalation study of SAR428926 in patients with advanced solid tumors.
|
||||
IMGN388 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [60] | ||||
| Patients Enrolled |
Advanced solid tumors.
|
||||
| Administration Dosage |
Doses ranging from 5 to 80 mg/m2, every 3 weeks, IV.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT00721669 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 dose-escalation study of IMGN388 in patients with solid tumors.
|
||||
| Primary Endpoint |
No evidence of activity was observed at the lowest doses (45 mg/m2) evaluated.
|
||||
| Other Endpoint |
No evidence of human anti-human antibody formation (data available for doses up to 60 mg/m2.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [61] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT00721669 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 dose-escalation study of IMGN388 in patients with solid tumors.
|
||||
| Primary Endpoint |
Five patients (breast,prostate,neuroendocrine,and 2 NSCLC) treated at doses 45 mg/m2 have acheived stable disease for 4 cycles.
|
||||
HKT-288 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [62] | ||||
| Patients Enrolled |
Advanced (metastatic or locally advanced) serous epithelial ovarian, serous fallopian tubal or serous primary peritoneal cancer or advanced clear cell or papillary renal cell carcinoma (RCC), who had received or were intolerant to all therapies known to confer clinical benefit for their disease and Eastern Cooperative Oncology Group (ECOG) performance status 2.
Click to Show/Hide
|
||||
| Administration Dosage |
HKT288 was administered intravenously (IV) every 3 weeks until patients experienced unacceptable toxicity or progressive disease (PD). The starting dose of 0.30 mg/kg was determined based on the highest nonseverely toxic dose in monkeys, which was 2 mg/kg IV weekly.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02947152 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1, multicenter, open-label dose escalation and expansion study of HKT288, administered intravenously in adult patients with advanced solid tumors, including epithelial ovarian cancer and renal cell carcinoma.
|
||||
| Primary Endpoint |
The best overall response on the 0.30 mg/kg cohort in patients with measurable disease was RECIST v1.1 stable disease in 3 patients and PD in 2 patients.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [63] | ||||
| Patients Enrolled |
Patients with advanced solid tumors, including epithelial ovarian cancer and renal cell carcinoma.
|
||||
| Administration Dosage |
Cadherin-6-targeting ADC iv at dose of 0.30, 0.75 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02947152 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1, multicenter, open-label dose escalation and expansion study of HKT288, administered intravenously in adult patients with advanced solid tumors, including epithelial ovarian cancer and renal cell carcinoma.
|
||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [66] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.00% (Day 45) | High CDH6 expression (CDH6+++) | ||
| Method Description |
CDH6-sulfo-DM4 induces efficient tumor cell killing in cell PDX models with CDH6 expression.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [66] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 45) | High CDH6 expression (CDH6+++) | ||
| Method Description |
CDH6-sulfo-DM4 induces efficient tumor cell killing in cell PDX models with CDH6 expression.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
BIIB-015 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [64] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT00674947 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 study of B2B015, a humanized, IgG1, DM4-conjugated, anti-cripto, monoclonal antibody, for the treatment of subjects with relapsed or refractory solid tumors.
|
||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [65] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 53.40% (Day 33) | High TDGF1 expression (TDGF1+++) | ||
| Method Description |
NCCIT cells were inoculated into 8-week old male athymic nude mice. MDA-MB-231 cells were inoculated into 7-week old female CB17 SCID mice. Calu-6,human non-small cell lung cancer cells (ATCC) were maintained in MEM Earless BSS/NEAA/10%FBS media without antibiotics and inoculated subcutaneously (SC) into the right flank of 9-week old female athymic nude mice. For the CT-3 tumour model,primary human colon tumour tissue was serially transplanted in vivo to establish a SC xenograft model. Cryopreserved tumour fragments were thawed and serially passaged SC in female SCID beige mice at 810 weeks old for two to five generations prior to implantation for studies.
Click to Show/Hide
|
||||
| In Vivo Model | MDA-MB-231 xenograft model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
Bispecific nBT062-natalizumab-DM4 [Investigative]
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [67] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 51.60% (Day 56) | Positive CD138 expression (CD138+++/++) | ||
| Method Description |
Bispecific nBT062-natalizumab-DM4 (4 mg/kg/week for three injections in total) induces efficient tumor cell killing in models of MAXF1322 mammary carcinoma cells with CD138 expression with high expression.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: MAXF 1322) | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [67] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.46 nM
|
Positive CD138 expression (CD138+++/++) | ||
| Method Description |
WT nBT062-DM4, stable nBT062-DM4, half nBT062-DM4 or bispecific nBT062-natalizumab-DM4 were added to the cells. Cells were incubated and after 5 days the viability was determined using the WST-1 cell proliferation assay.
|
||||
| In Vitro Model | Plasma cell myeloma | NCI-H929 cells | CVCL_1600 | ||
Anti-DOG1-DM4-ADC [Investigative]
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.42% (Day 50) | Positive DOG1 expression (DOG1 +++/++) | ||
| Method Description |
5 mg/kg anti-DOG1 ADC and control (PBS) were administered Q3Dx3 via tail vein injection to mice once every three days for a total of three doses.
|
||||
| In Vivo Model | Gastrointestinal stromal tumor (GIST) PDX model | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.90% (Day 50) | Positive DOG1 expression (DOG1 +++/++) | ||
| Method Description |
10 mg/kg anti-DOG1 ADC and control (PBS) were administered Q3Dx3 via tail vein injection to mice once every three days for a total of three doses.
|
||||
| In Vivo Model | Gastrointestinal stromal tumor (GIST) PDX model | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 69.69% (Day 17) | Positive DOG1 expression (DOG1 +++/++) | ||
| Method Description |
Nude mice were used to establish cell line derived xenograft (CDX) models. After tumor inoculation, the tumor bearing mice were intravenously injected with naked anti-DOG1 antibody or a 5 mg/kg Q3Dx3 dose of the anti-DOG1 ADC.
|
||||
| In Vivo Model | Kyse-410 CDX model | ||||
| In Vitro Model | Esophageal squamous cell carcinoma | KYSE-410 cells | CVCL_1352 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 83.30% (Day 25) | Positive DOG1 expression (DOG1 +++/++) | ||
| Method Description |
Nude mice were used to establish cell line derived xenograft (CDX) models. After tumor inoculation, the tumor bearing mice were intravenously injected with naked anti-DOG1 antibody or a 5 mg/kg Q3Dx3 dose of the anti-DOG1 ADC.
|
||||
| In Vivo Model | HepG2 CDX model | ||||
| In Vitro Model | Hepatoblastoma | Hep-G2 cells | CVCL_0027 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 89.32% (Day 25) | Positive DOG1 expression (DOG1 +++/++) | ||
| Method Description |
Nude mice were used to establish cell line derived xenograft (CDX) models. After tumor inoculation, the tumor bearing mice were intravenously injected with naked anti-DOG1 antibody or a 5 mg/kg Q3Dx3 dose of the anti-DOG1 ADC.
|
||||
| In Vivo Model | HT-29 CDX model | ||||
| In Vitro Model | Colon adenocarcinoma | HT-29 cells | CVCL_0320 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 92.04% (Day 25) | Positive DOG1 expression (DOG1 +++/++) | ||
| Method Description |
Nude mice were used to establish cell line derived xenograft (CDX) models. After tumor inoculation, the tumor bearing mice were intravenously injected with naked anti-DOG1 antibody or a 10 mg/kg Q3Dx3 dose of the anti-DOG1 ADC.
|
||||
| In Vivo Model | HepG2 CDX model | ||||
| In Vitro Model | Hepatoblastoma | Hep-G2 cells | CVCL_0027 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 92.11% (Day 21) | Positive DOG1 expression (DOG1 +++/++) | ||
| Method Description |
Nude mice were used to establish cell line derived xenograft (CDX) models. After tumor inoculation, the tumor bearing mice were intravenously injected with naked anti-DOG1 antibody or a 5 mg/kg Q3Dx3 dose of the anti-DOG1 ADC.
|
||||
| In Vivo Model | MGC-803 CDX model | ||||
| In Vitro Model | Gastric cancer | MGC-803 cells | CVCL_5334 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.53% (Day 17) | Positive DOG1 expression (DOG1 +++/++) | ||
| Method Description |
Nude mice were used to establish cell line derived xenograft (CDX) models. After tumor inoculation, the tumor bearing mice were intravenously injected with naked anti-DOG1 antibody or a 10 mg/kg Q3Dx3 dose of the anti-DOG1 ADC.
|
||||
| In Vivo Model | Kyse-410 CDX model | ||||
| In Vitro Model | Esophageal squamous cell carcinoma | KYSE-410 cells | CVCL_1352 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.24% (Day 21) | Positive DOG1 expression (DOG1 +++/++) | ||
| Method Description |
Nude mice were used to establish cell line derived xenograft (CDX) models. After tumor inoculation, the tumor bearing mice were intravenously injected with naked anti-DOG1 antibody or a 10 mg/kg Q3Dx3 dose of the anti-DOG1 ADC.
|
||||
| In Vivo Model | MGC-803 CDX model | ||||
| In Vitro Model | Gastric cancer | MGC-803 cells | CVCL_5334 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.67% (Day 25) | Positive DOG1 expression (DOG1 +++/++) | ||
| Method Description |
Nude mice were used to establish cell line derived xenograft (CDX) models. After tumor inoculation, the tumor bearing mice were intravenously injected with naked anti-DOG1 antibody or a 10 mg/kg Q3Dx3 dose of the anti-DOG1 ADC.
|
||||
| In Vivo Model | HT-29 CDX model | ||||
| In Vitro Model | Colon adenocarcinoma | HT-29 cells | CVCL_0320 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
8.37 nM
|
Positive DOG1 expression (DOG1 +++/++) | ||
| Method Description |
Cancer cells were treated with anti-DOG1-DM4-ADC or naked anti-DOG1 antibody (as control) at different concentrations for 72 hours.Viable cell counts were determined with RTCA assays and the IC 50 values were calculated.
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
8.87 nM
|
Positive DOG1 expression (DOG1 +++/++) | ||
| Method Description |
Cancer cells were treated with anti-DOG1-DM4-ADC or naked anti-DOG1 antibody (as control) at different concentrations for 72 hours.Viable cell counts were determined with RTCA assays and the IC 50 values were calculated.
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells (Imatinib resistant) | CVCL_7044 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
9.91 nM
|
Positive DOG1 expression (DOG1 +++/++) | ||
| Method Description |
Cancer cells were treated with anti-DOG1-DM4-ADC or naked anti-DOG1 antibody (as control) at different concentrations for 72 hours.Viable cell counts were determined with RTCA assays and the IC 50 values were calculated.
|
||||
| In Vitro Model | Colon adenocarcinoma | HT-29 cells | CVCL_0320 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
12.11 nM
|
Positive DOG1 expression (DOG1 +++/++) | ||
| Method Description |
Cancer cells were treated with anti-DOG1-DM4-ADC or naked anti-DOG1 antibody (as control) at different concentrations for 72 hours.Viable cell counts were determined with RTCA assays and the IC 50 values were calculated.
|
||||
| In Vitro Model | Esophageal squamous cell carcinoma | KYSE-410 cells | CVCL_1352 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
17.92 nM
|
Positive DOG1 expression (DOG1 +++/++) | ||
| Method Description |
Cancer cells were treated with anti-DOG1-DM4-ADC or naked anti-DOG1 antibody (as control) at different concentrations for 72 hours.Viable cell counts were determined with RTCA assays and the IC 50 values were calculated.
|
||||
| In Vitro Model | Gastric cancer | MGC-803 cells | CVCL_5334 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
24.56 nM
|
Positive DOG1 expression (DOG1 +++/++) | ||
| Method Description |
Cancer cells were treated with anti-DOG1-DM4-ADC or naked anti-DOG1 antibody (as control) at different concentrations for 72 hours.Viable cell counts were determined with RTCA assays and the IC 50 values were calculated.
|
||||
| In Vitro Model | Hepatoblastoma | Hep-G2 cells | CVCL_0027 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
33.19 nM
|
Positive DOG1 expression (DOG1 +++/++) | ||
| Method Description |
Cancer cells were treated with anti-DOG1-DM4-ADC or naked anti-DOG1 antibody (as control) at different concentrations for 72 hours.Viable cell counts were determined with RTCA assays and the IC 50 values were calculated.
|
||||
| In Vitro Model | Adult hepatocellular carcinoma | HCCLM3 cells | CVCL_6832 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
39.99 nM
|
Positive DOG1 expression (DOG1 +++/++) | ||
| Method Description |
Cancer cells were treated with anti-DOG1-DM4-ADC or naked anti-DOG1 antibody (as control) at different concentrations for 72 hours.Viable cell counts were determined with RTCA assays and the IC 50 values were calculated.
|
||||
| In Vitro Model | Colon carcinoma | HCT 116 cells | CVCL_0291 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [68] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 200 nM | Negative DOG1 expression (DOG1 -) | ||
| Method Description |
Cancer cells were treated with anti-DOG1-DM4-ADC or naked anti-DOG1 antibody (as control) at different concentrations for 72 hours.Viable cell counts were determined with RTCA assays and the IC 50 values were calculated.
|
||||
| In Vitro Model | Colon adenocarcinoma | LoVo cells | CVCL_0399 | ||
Stable nBT062-DM4 [Investigative]
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [67] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 56) | Positive CD138 expression (CD138+++/++) | ||
| Method Description |
Stable nBT062-DM4 (4 mg/kg/week for three injections in total) induces efficient tumor cell killing in models of MAXF1322 mammary carcinoma cells with CD138 expression with high expression.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: MAXF 1322) | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [67] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.14 nM±0.01 nM
|
Positive CD138 expression (CD138+++/++) | ||
| Method Description |
WT nBT062-DM4, stable nBT062-DM4, half nBT062-DM4 or bispecific nBT062-natalizumab-DM4 were added to the cells. Cells were incubated and after 5 days the viability was determined using the WST-1 cell proliferation assay.
|
||||
| In Vitro Model | Plasma cell myeloma | NCI-H929 cells | CVCL_1600 | ||
Half nBT062-DM4 [Investigative]
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [67] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 56) | Positive CD138 expression (CD138+++/++) | ||
| Method Description |
Half nBT062-DM4 (4 mg/kg/week for three injections in total) induces efficient tumor cell killing in models of MAXF1322 mammary carcinoma cells with CD138 expression with high expression.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: MAXF 1322) | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [67] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.085 nM±0.005 nM
|
Positive CD138 expression (CD138+++/++) | ||
| Method Description |
WT nBT062-DM4, stable nBT062-DM4, half nBT062-DM4 or bispecific nBT062-natalizumab-DM4 were added to the cells. Cells were incubated and after 5 days the viability was determined using the WST-1 cell proliferation assay.
|
||||
| In Vitro Model | Plasma cell myeloma | NCI-H929 cells | CVCL_1600 | ||
Wild type nBT062-DM4 [Investigative]
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [67] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 56) | Positive CD138 expression (CD138+++/++) | ||
| Method Description |
Wild type nBT062-DM4 (4 mg/kg/week for three injections in total) induces efficient tumor cell killing in models of MAXF1322 mammary carcinoma cells with CD138 expression with high expression.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: MAXF 1322) | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [67] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM±0.02 nM
|
Positive CD138 expression (CD138+++/++) | ||
| Method Description |
WT nBT062-DM4, stable nBT062-DM4, half nBT062-DM4 or bispecific nBT062-natalizumab-DM4 were added to the cells. Cells were incubated and after 5 days the viability was determined using the WST-1 cell proliferation assay.
|
||||
| In Vitro Model | Plasma cell myeloma | NCI-H929 cells | CVCL_1600 | ||
F105-SSNPP-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 37) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Female athymic rats were inoculated with 5x106 HT29 cells subcutancously on the rear flank area. The animals were stratified into 13 groups, 6 animals per group based on a mean tumor volume for each group of approximately 250 mm3. On the day of grouping (day 7) each group received its initial dosing (175 ug/kg).
|
||||
| In Vivo Model | HT-29 CDX model | ||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
CNTO95-SSNPP-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 37) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Female athymic rats were inoculated with 5x106 HT29 cells subcutancously on the rear flank area. The animals were stratified into 13 groups, 6 animals per group based on a mean tumor volume for each group of approximately 250 mm3. On the day of grouping (day 7) each group received its initial dosing (175 ug/kg).
|
||||
| In Vivo Model | HT-29 CDX model | ||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 2 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 71.21% (Day 24) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Nine-week-old athymic nude rats were subcutaneously inoculated with A375.S2human melanoma cells. ADC (5 mg/kg) andappropriate control compounds were intravenously injected (three injection every other day inthe first week followed by one injection per week for two weeks.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.15% (Day 24) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Nine-week-old athymic nude rats were subcutaneously inoculated with A375.S2human melanoma cells. ADC (10 mg/kg) andappropriate control compounds were intravenously injected (three injection every other day inthe first week followed by one injection per week for two weeks.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.10% (Day 49) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
The rear flank region of female athymic rats were implanted with 5x106 cells subcutaneously (0.2 ml of25 x106 cells/ml) on the rear flank area. When mean tumor volumes reached to 250 mm3, dosed intravenously (15 mg/kg) on days 17 and 29 after tumor cell injection.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.00 ug/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were seeded into white 96-well tissue culture plates (5000 cells/well)in culture medium and incubated for 16 hrs. Serial dilutions of immunoconjugates were added toeach appropraite wells (0-20 ug/ml). Tissue culture plates were incubated at 37°C for 96 hrs.
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 2 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.00 ug/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were seeded into white 96-well tissue culture plates (5000 cells/well)in culture medium and incubated for 16 hrs. Serial dilutions of immunoconjugates were added toeach appropraite wells (0-20 ug/ml). Tissue culture plates were incubated at 37°C for 96 hrs.
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 3 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.20 ug/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were seeded into white 96-well tissue culture plates (5000 cells/well)in culture medium and incubated for 16 hrs. Serial dilutions of immunoconjugates were added toeach appropraite wells (0-20 ug/ml). Tissue culture plates were incubated at 37°C for 96 hrs.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.50 ug/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were seeded into white 96-well tissue culture plates (5000 cells/well)in culture medium and incubated for 16 hrs. Serial dilutions of immunoconjugates were added toeach appropraite wells (0-20 ug/ml). Tissue culture plates were incubated at 37°C for 96 hrs.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.19 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 6 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.27 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.27 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | A2780 cells | CVCL_0134 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.29 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 9 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.30 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | A2780 cells | CVCL_0134 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.42 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
NOV0712-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [70] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 13) | Negative CDH6 expression (CDH6-) | ||
| Method Description |
The CDH6-negative human ovarian tumor cell line ES-2 was subcutaneously inoculated at a dose of 1,000,000 cells to the right flank region of each female nude mouse (Day 0). On the day 7 of grouping, the antibody-drug conjugate NOV0712-DM4 was intravenously administered at doses of 1 mg/kg to thetail of each mouse.
|
||||
| In Vivo Model | ES-2 CDX model | ||||
| In Vitro Model | Ovarian clear cell adenocarcinoma | ES-2 cells | CVCL_3509 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [70] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 13) | Negative CDH6 expression (CDH6-) | ||
| Method Description |
The CDH6-negative human ovarian tumor cell line ES-2 was subcutaneously inoculated at a dose of 1,000,000 cells to the right flank region of each female nude mouse (Day 0). On the day 7 of grouping, the antibody-drug conjugate NOV0712-DM4 was intravenously administered at doses of 3 mg/kg to thetail of each mouse.
|
||||
| In Vivo Model | ES-2 CDX model | ||||
| In Vitro Model | Ovarian clear cell adenocarcinoma | ES-2 cells | CVCL_3509 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [70] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 3.10% (Day 28) | Positive CDH6 expression (CDH6+++/++) | ||
| Method Description |
The CDH6-positive human ovarian tumor cell line OVCAR-3 was subcutane-ously inoculated at a dose of 10,000,000 cells to the right flank region of each female nude mouse (Day 0). On the day 22 of grouping, theantibody-drug conjugate NOV0712-DM4 was intravenously administered at doses of 1 mg/kg to the tail of each mouse.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [70] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 6.77% (Day 21) | Positive CDH6 expression (CDH6+++/++) | ||
| Method Description |
The CDH6-positive human ovarian tumor cell line PA-1 was subcutaneously inoculatedat a dose of 8,500,000 cells to the right flank region of eachfemale nude mouse (Day 0). On the day 11 of grouping, the antibody-drug conjugate NOV0712-DM4 was intravenously administered at doses of 3 mg/kg to the tail of each mouse.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [70] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 9.52% (Day 23) | Positive CDH6 expression (CDH6+++/++) | ||
| Method Description |
The CDH6-positive human renal cell tumor cell line786-0 was subcutaneously inoculated at a dose of 5,000,000 cells to the right flank regionof each male SCID mouse (Day 0). On the day 20 of grouping, the anti-body-drug conjugate NOV0712-DM4 was intravenously administered at doses of 1 mg/kg to thetail of each mouse.
|
||||
| In Vivo Model | 786-O CDX model | ||||
| In Vitro Model | Renal cell carcinoma | 786-O cells | CVCL_1051 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [70] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 12.03% (Day 21) | Positive CDH6 expression (CDH6+++/++) | ||
| Method Description |
The CDH6-positive human ovarian tumor cell line PA-1 was subcutaneously inoculatedat a dose of 8,500,000 cells to the right flank region of eachfemale nude mouse (Day 0). On the day 11 of grouping, the antibody-drug conjugate NOV0712-DM4 was intravenously administered at doses of 1 mg/kg to the tail of each mouse.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [70] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 23.91% (Day 23) | Positive CDH6 expression (CDH6+++/++) | ||
| Method Description |
The CDH6-positive human renal cell tumor cell line786-0 was subcutaneously inoculated at a dose of 5,000,000 cells to the right flank regionof each male SCID mouse (Day 0). On the day 20 of grouping, the anti-body-drug conjugate NOV0712-DM4 was intravenously administered at doses of 3 mg/kg to thetail of each mouse.
|
||||
| In Vivo Model | 786-O CDX model | ||||
| In Vitro Model | Renal cell carcinoma | 786-O cells | CVCL_1051 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [70] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 30.02% (Day 24) | Positive CDH6 expression (CDH6+++/++) | ||
| Method Description |
The CDH6-positive human renal cell tumor cell line 786-O was subcutaneously inoculated at a dose of 5,000,000 cells to the right flank regionof each male SCID mouse (Day 0). On the day 18 of grouping, NOV0712-DM4 was intravenously administered at a dose of 3 mg/kg to the tail of each mouse.
|
||||
| In Vivo Model | 786-O CDX model | ||||
| In Vitro Model | Renal cell carcinoma | 786-O cells | CVCL_1051 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [70] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 72.30% (Day 28) | Positive CDH6 expression (CDH6+++/++) | ||
| Method Description |
The CDH6-positive human ovarian tumor cell line OVCAR-3 was subcutane-ously inoculated at a dose of 10,000,000 cells to the right flank region of each female nude mouse (Day 0). On the day 22 of grouping, theantibody-drug conjugate NOV0712-DM4 was intravenously administered at doses of 3 mg/kg to the tail of each mouse.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [70] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00-10.00 nM
|
Positive CDH6 expression (CDH6+++/++) | ||
| Method Description |
CDH6-positive human ovarian tumor cell line PA-1 was seeded over a 96-well plate at 2,000 cells/100 L/well in MEM medium supplemented with 10% FBS, and the cells were then cultured overnight. On the next day, each of the 4 humanized hG019-drug conjugates or NOV0712-DM4 was added to the cells.
|
||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
muFR1-13-PEG4-Mal-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 18.22% (Day 22) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
PEG4Mal-DM4 conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 3 post cell inoculation with 10 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.15 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
FOLR1-expressing SKOV3 cells were incubated with varying concentrations of anti FOLR1 antibodies or their conjugates and processed.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
muFR1-23-PEG4-Mal-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 20.03% (Day 22) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
PEG4Mal-DM4 conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 3 post cell inoculation with 10 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.13 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
FOLR1-expressing SKOV3 cells were incubated with varying concentrations of anti FOLR1 antibodies or their conjugates and processed.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
huMov19-PEG4-Mal-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 24.92% (Day 22) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
PEG4Mal-DM4 conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 2.5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 46.37% (Day 22) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
PEG4Mal-DM4 conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 5 mg/kg ofone of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 68.53% (Day 22) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
PEG4Mal-DM4 conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 3 post cell inoculation with 10 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 76.02% (Day 22) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
PEG4Mal-DM4 conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 10 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.21% (Day 22) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
PEG4Mal-DM4 conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 10 mg/kg (every week, three weeks) of one of the conjugates listed above or with PBS only.
Click to Show/Hide
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 30) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Mice were randomized by body weight into treatment groups and reated either singly (SPDB conjugates) on day 3 post cell inoculation, or three times weekly on days 3, 10, and 17 post KB cell inoculation with 10 mg/kg of a conjugate.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.15 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
FOLR1-expressing SKOV3 cells were incubated with varying concentrations of anti FOLR1 antibodies or their conjugates and processed.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
huMov19-3-sulfo-Mal-DM4 (DAR 8.23) [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 26.41% (Day 20) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 1.1 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 39.00% (Day 20) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 2.1 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 85.77% (Day 20) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 4.5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
HuIgG1-SPDB-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
30.00% (Day 20)
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Animals with established tumors of about 130 mm3 were treated with intravenous single injection of the M9346A-DM conjugates at 50 mg/kg, equivalent to 82 g conjugated maytansinoid per kg The conjugates were injected on day 4 after cell inoculation.
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
muFR1-22-PEG4-Mal-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 30.33% (Day 22) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
PEG4Mal-DM4 conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 3 post cell inoculation with 10 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.14 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
FOLR1-expressing SKOV3 cells were incubated with varying concentrations of anti FOLR1 antibodies or their conjugates and processed.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
Anti-KIT 9P3?SPDB-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 31.88% (Day 14) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with Kasumi-1 cells. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (1 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | Kasumi-1 CDX model | ||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 91.94% (Day 35) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with Kasumi-1 cells. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (10 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | HMC-1 CDX model | ||||
| In Vitro Model | Mast cell leukemia | HMC-1 cells | CVCL_0003 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.00% (Day 50) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.00% (Day 43) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST430 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.09% (Day 14) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with Kasumi-1 cells. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | Kasumi-1 CDX model | ||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 50) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
65.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
75.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
84.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H889 cells | CVCL_1598 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
88.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1930 cells | CVCL_1507 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
92.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute megakaryoblastic leukemia | CMK-11-5 cells | CVCL_0217 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
94.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Erythroleukemia | HEL 92.1.7 cells | CVCL_2481 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
94.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
98.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute megakaryoblastic leukemia | M-07e cells | CVCL_2106 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Adult acute myeloid leukemia | SKNO-1 cells | CVCL_2196 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
100.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
100.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | OCI-M1 cells | CVCL_2149 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
100.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Essential thrombocythemia | UKE-1 cells | CVCL_0104 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
100.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.05 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.07 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute megakaryoblastic leukemia | CMK-11-5 cells | CVCL_0217 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.11 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute megakaryoblastic leukemia | M-07e cells | CVCL_2106 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.13 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | OCI-M1 cells | CVCL_2149 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.17 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 20 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.17 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 21 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.30 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1930 cells | CVCL_1507 | ||
| Experiment 22 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.83 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 23 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.91 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 24 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
1.26 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Erythroleukemia | HEL 92.1.7 cells | CVCL_2481 | ||
| Experiment 25 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
1.47 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H889 cells | CVCL_1598 | ||
| Experiment 26 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
1.60 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Adult acute myeloid leukemia | SKNO-1 cells | CVCL_2196 | ||
| Experiment 27 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
2.77 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 28 Reporting the Activity Date of This ADC | [71] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
5.60 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Essential thrombocythemia | UKE-1 cells | CVCL_0104 | ||
CDH6-SPDB-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [66] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.00% (Day 45) | High CDH6 expression (CDH6+++) | ||
| Method Description |
CDH6-SPDB-DM4 induces efficient tumor cell killing in cell PDX models with CDH6 expression.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian cancer | Ovarian cancer cells | Homo sapiens | ||
| Experiment 2 Reporting the Activity Date of This ADC | [66] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 50.00% (Day 45) | High CDH6 expression (CDH6+++) | ||
| Method Description |
CDH6-SPDB-DM4 induces efficient tumor cell killing in cell PDX models with CDH6 expression.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian cancer | Ovarian cancer cells | Homo sapiens | ||
| Experiment 3 Reporting the Activity Date of This ADC | [66] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 45) | High CDH6 expression (CDH6+++) | ||
| Method Description |
CDH6-SPDB-DM4 induces efficient tumor cell killing in cell PDX models with CDH6 expression.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
F105-SSNPB-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.81% (Day 37) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Female athymic rats were inoculated with 5x106 HT29 cells subcutancously on the rear flank area. The animals were stratified into 13 groups, 6 animals per group based on a mean tumor volume for each group of approximately 250 mm3. On the day of grouping (day 7) each group received its initial dosing (175 ug/kg).
|
||||
| In Vivo Model | HT-29 CDX model | ||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
huMov19-3-sulfo-Mal-DM4 (DAR 3.7) [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 47.64% (Day 20) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 2.5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 59.21% (Day 20) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 83.57% (Day 20) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 10 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
muFR1-9-PEG4-Mal-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 47.84% (Day 22) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
PEG4Mal-DM4 conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 3 post cell inoculation with 10 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.22 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
FOLR1-expressing SKOV3 cells were incubated with varying concentrations of anti FOLR1 antibodies or their conjugates and processed.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
BT062-SPDB-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [72] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 56.56% (Day 26) | |||
| Method Description |
The inhibitory activity of nBT062-SPDB-DM4 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 100g/kg.
|
||||
| In Vivo Model | SCID-hu MM model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [72] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.92% (Day 26) | |||
| Method Description |
The inhibitory activity of nBT062-SPDB-DM4 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 250g/kg.
|
||||
| In Vivo Model | SCID-hu MM model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [72] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.00% (Day 26) | |||
| Method Description |
The inhibitory activity of nBT062-SPDB-DM4 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 450g/kg.
|
||||
| In Vivo Model | SCID-hu MM model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
Tmab-SSNPP-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [73] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.60% (Day 10) | High HER2 expression (HER2+++) | ||
| Method Description |
Mice bearing mammary tumor transplants from the MMTV-HER2 Fo5 line were given a single iv injection (10 mg/kg) of Tmab-SPP-DM1, Tmab-SSNPP-DM3, Tmab-SSNPP-DM4, Tmab-MCC-DM1, or vehicle (n=7 mice per group), and tumor growth was monitored for 25 days.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
CNTO95-SSNPB-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 82.51% (Day 37) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Female athymic rats were inoculated with 5x106 HT29 cells subcutancously on the rear flank area. The animals were stratified into 13 groups, 6 animals per group based on a mean tumor volume for each group of approximately 250 mm3. On the day of grouping (day 7) each group received its initial dosing (175 ug/kg).
|
||||
| In Vivo Model | HT-29 CDX model | ||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.24 ug/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were seeded into white 96-well tissue culture plates (5000 cells/well)in culture medium and incubated for 16 hrs. Serial dilutions of immunoconjugates were added toeach appropraite wells (0-20 ug/ml). Tissue culture plates were incubated at 37°C for 96 hrs.
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 2 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.30 ug/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were seeded into white 96-well tissue culture plates (5000 cells/well)in culture medium and incubated for 16 hrs. Serial dilutions of immunoconjugates were added toeach appropraite wells (0-20 ug/ml). Tissue culture plates were incubated at 37°C for 96 hrs.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.21 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 4 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.27 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | A2780 cells | CVCL_0134 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.34 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
Mirvetuximab-SPDB-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
83.00% (Day 32)
|
High FOLR1 expression (FOLR1+++; 1,300,000 FOLR1 molecules/cell) | ||
| Method Description |
Animals with established tumors of about 130 mm3 were treated with intravenous single injection of the M9346A-DM conjugates at 2.5±0.2 mg/kg, equivalent to 51±3 ug conjugated maytansinoid per kg The conjugates were injected on day 7 after cell inoculation.
|
||||
| In Vivo Model | Ovarian carcinoma Igrov-1 CDX model | ||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
92.22% (Day 26)
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Animals with established tumors of about 130 mm3 were treated with intravenous single injection of the M9346A-DM conjugates at 2.5±0.2 mg/kg, equivalent to 51±3 ug conjugated maytansinoid per kg The conjugates were injected on day 20 after cell inoculation.
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07±0.02 nM
|
High FOLR1 expression (FOLR1+++; 1,300,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.08±0.01 nM
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.11±0.08 nM
|
Moderate FOLR1 expression (FOLR1++; 290,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Gestational choriocarcinoma | JEG-3 cells | CVCL_0363 | ||
1959-sss/DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [74] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 89.57% (Day 30) | High LGALS3BP expression (LGALS3BP +++) | ||
| Method Description |
Gch6 cell xenograft mice were intravenously treated with 10 mg/kg 1959-sss/DM4 twice weekly for a total of three injections.
|
||||
| In Vivo Model | Gch6 CDX model | ||||
| In Vitro Model | Glioblastoma | Gch6 cells | Homo sapiens | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [74] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.02 nM
|
High LGALS3BP expression (LGALS3BP +++) | ||
| Method Description |
In vitro cytotoxicity of ADCs against a panel of four multiple human glioblastoma cell lines.
|
||||
| In Vitro Model | Glioblastoma | Gch14 cells | Homo sapiens | ||
| Experiment 2 Reporting the Activity Date of This ADC | [74] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.57 nM
|
High LGALS3BP expression (LGALS3BP +++) | ||
| Method Description |
In vitro cytotoxicity of ADCs against a panel of four multiple human glioblastoma cell lines.
|
||||
| In Vitro Model | Glioblastoma | Gch6 cells | Homo sapiens | ||
huMov19-sulfo-SPDB-DM4 (DAR6.8) [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.72% (Day 17) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 2.5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 17) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
huMov19-SPDB-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.72% (Day 30) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Mice were randomized by body weight into treatment groups and reated either singly (SPDB conjugates) on day 3 post cell inoculation, or three times weekly on days 3, 10, and 17 post KB cell inoculation with 5 mg/kg of a conjugate.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.08% (Day 17) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 2.5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 17) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.20 nM
|
Moderate FOLR1 expression (FOLR1 ++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Gestational choriocarcinoma | JEG-3 cells | CVCL_0363 | ||
huMov19-sulfo-SPDB-DM4 (DAR3.8) [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.08% (Day 17) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 2.5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 17) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.30 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.00 nM
|
Moderate FOLR1 expression (FOLR1 ++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Gestational choriocarcinoma | JEG-3 cells | CVCL_0363 | ||
nBT062-SPDB-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.40% (Day 20) | Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
MOLP-8 cells (1.5x107 cells per mouse) suspended in a 50:50 mixture of serum free media and matrigel were injected subcutaneously in the area under the right shoulder in 100 ul. Nine groups (n=6) were treated with a single intravenous injection of ADCs, each at doses of 250 ug/kg.
|
||||
| In Vivo Model | MOLP-8 CDX model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM-1.00 nM
|
Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
CD138+ MOLP-8 cells were seeded in flat bottom plates at 3000 cells/well. CD138- BJAB control cells were seeded at 1000 cells/weli. The cells were treated with nBT062-SPDB-DM4nBT062-SPP-DM1 or nBT062-SMCC-DM1 at different concentrations for five days.
|
||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Negative CD138 expression (CD138 -) | ||
| Method Description |
CD138+ MOLP-8 cells were seeded in flat bottom plates at 3000 cells/well. CD138- BJAB control cells were seeded at 1000 cells/weli. The cells were treated with nBT062-SPDB-DM4nBT062-SPP-DM1 or nBT062-SMCC-DM1 at different concentrations for five days.
|
||||
| In Vitro Model | Burkitt lymphoma | BJAB cells | CVCL_5711 | ||
M9346A-SPDB-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 20) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OVCAR-3 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
M9346A-sulfo-Mal-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 20) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OVCAR-3 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
huDS6v1.01-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [75] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 37) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
The in vivo activity of huDS6v1.01-DM4 was tested onthe HPAC pancreatic model.HPAC cells were inoculated on Day 0, and immunoconjugate treatments were given on day13 (20 ug/kg). PBS control animals were euthanized once tumor vol-umes exceeded 1000 mm3.
|
||||
| In Vivo Model | HPAC CDX model | ||||
| In Vitro Model | Pancreatic adenocarcinoma | HPAC cells | CVCL_3517 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [75] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 10.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of huDS6v1.01-DM4 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Anti-FGFR2 mAb-12433 SPDB-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [76] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 35) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 233 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 5 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [76] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.80 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Anti-FGFR2/4 mAb-12425 SPDB-DM4 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [76] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Anti-FGFR2/4 mAb-12422 SPDB-DM4 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [76] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Farletuzumab-sulfo SPDB-DM4 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [77] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.09 nM
|
High FOLR1 expression(FOLR1+++) | ||
| Method Description |
Cells were sub-cultured and seeded at 5,000 cells/well in complete growth medium in 96-well tissue culture plates, and incubated at 37°C, 5% CO2 overnight. Test reagents were serially diluted and added to the cell plates (initial concentration of 100 nM). Plates were incubated at 37°C, 5% CO2 for an additional 3 d.
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [77] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.49 nM
|
Moderate FOLR1 expression(FOLR1++) | ||
| Method Description |
Cells were sub-cultured and seeded at 5,000 cells/well in complete growth medium in 96-well tissue culture plates, and incubated at 37°C, 5% CO2 overnight. Test reagents were serially diluted and added to the cell plates (initial concentration of 100 nM). Plates were incubated at 37°C, 5% CO2 for an additional 3 d.
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [77] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.52 nM
|
Moderate FOLR1 expression(FOLR1++) | ||
| Method Description |
Cells were sub-cultured and seeded at 5,000 cells/well in complete growth medium in 96-well tissue culture plates, and incubated at 37°C, 5% CO2 overnight. Test reagents were serially diluted and added to the cell plates (initial concentration of 100 nM). Plates were incubated at 37°C, 5% CO2 for an additional 3 d.
|
||||
| In Vitro Model | Lung non-small cell carcinoma | NCI-H2110 cells | CVCL_1530 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [77] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.85 nM
|
Low FOLR1 expression(FOLR1+) | ||
| Method Description |
Cells were sub-cultured and seeded at 5,000 cells/well in complete growth medium in 96-well tissue culture plates, and incubated at 37°C, 5% CO2 overnight. Test reagents were serially diluted and added to the cell plates (initial concentration of 100 nM). Plates were incubated at 37°C, 5% CO2 for an additional 3 d.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [77] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.28 nM
|
Negative FOLR1 expression(FOLR1-) | ||
| Method Description |
Cells were sub-cultured and seeded at 5,000 cells/well in complete growth medium in 96-well tissue culture plates, and incubated at 37°C, 5% CO2 overnight. Test reagents were serially diluted and added to the cell plates (initial concentration of 100 nM). Plates were incubated at 37°C, 5% CO2 for an additional 3 d.
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
muFR1-21-PEG4-Mal-DM4 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
FOLR1-expressing SKOV3 cells were incubated with varying concentrations of anti FOLR1 antibodies or their conjugates and processed.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
huFR1-21-PEG4-Mal-DM4 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
FOLR1-expressing SKOV3 cells were incubated with varying concentrations of anti FOLR1 antibodies or their conjugates and processed.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
CNTO95-SPDB-DM4 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.24 ug/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were seeded into white 96-well tissue culture plates (5000 cells/well)in culture medium and incubated for 16 hrs. Serial dilutions of immunoconjugates were added toeach appropraite wells (0-20 ug/ml). Tissue culture plates were incubated at 37°C for 96 hrs.
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 2 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.30 ug/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were seeded into white 96-well tissue culture plates (5000 cells/well)in culture medium and incubated for 16 hrs. Serial dilutions of immunoconjugates were added toeach appropraite wells (0-20 ug/ml). Tissue culture plates were incubated at 37°C for 96 hrs.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.21 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 4 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.27 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | A2780 cells | CVCL_0134 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.34 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
CNTO95-SPP-DM4 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.00 ug/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were seeded into white 96-well tissue culture plates (5000 cells/well)in culture medium and incubated for 16 hrs. Serial dilutions of immunoconjugates were added toeach appropraite wells (0-20 ug/ml). Tissue culture plates were incubated at 37°C for 96 hrs.
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 2 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.50 ug/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were seeded into white 96-well tissue culture plates (5000 cells/well)in culture medium and incubated for 16 hrs. Serial dilutions of immunoconjugates were added toeach appropraite wells (0-20 ug/ml). Tissue culture plates were incubated at 37°C for 96 hrs.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.29 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 4 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.30 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | A2780 cells | CVCL_0134 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [69] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.42 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
References
