Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0GZGHN
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
SAR-566658
|
|||||
| Synonyms |
Anti-CA6-DM4-immunoconjugate-SAR566658; Anti-CA6-antibody-drug-conjugate; HuDS6-DM4; SAR 566658; SAR-566658; SAR56 6658; SAR566658; huDS6-DM4
Click to Show/Hide
|
|||||
| Organization |
Sanofi; ImmunoGen, Inc.
|
|||||
| Drug Status |
Phase 2 (Terminated)
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
1
|
|||||
| Structure |
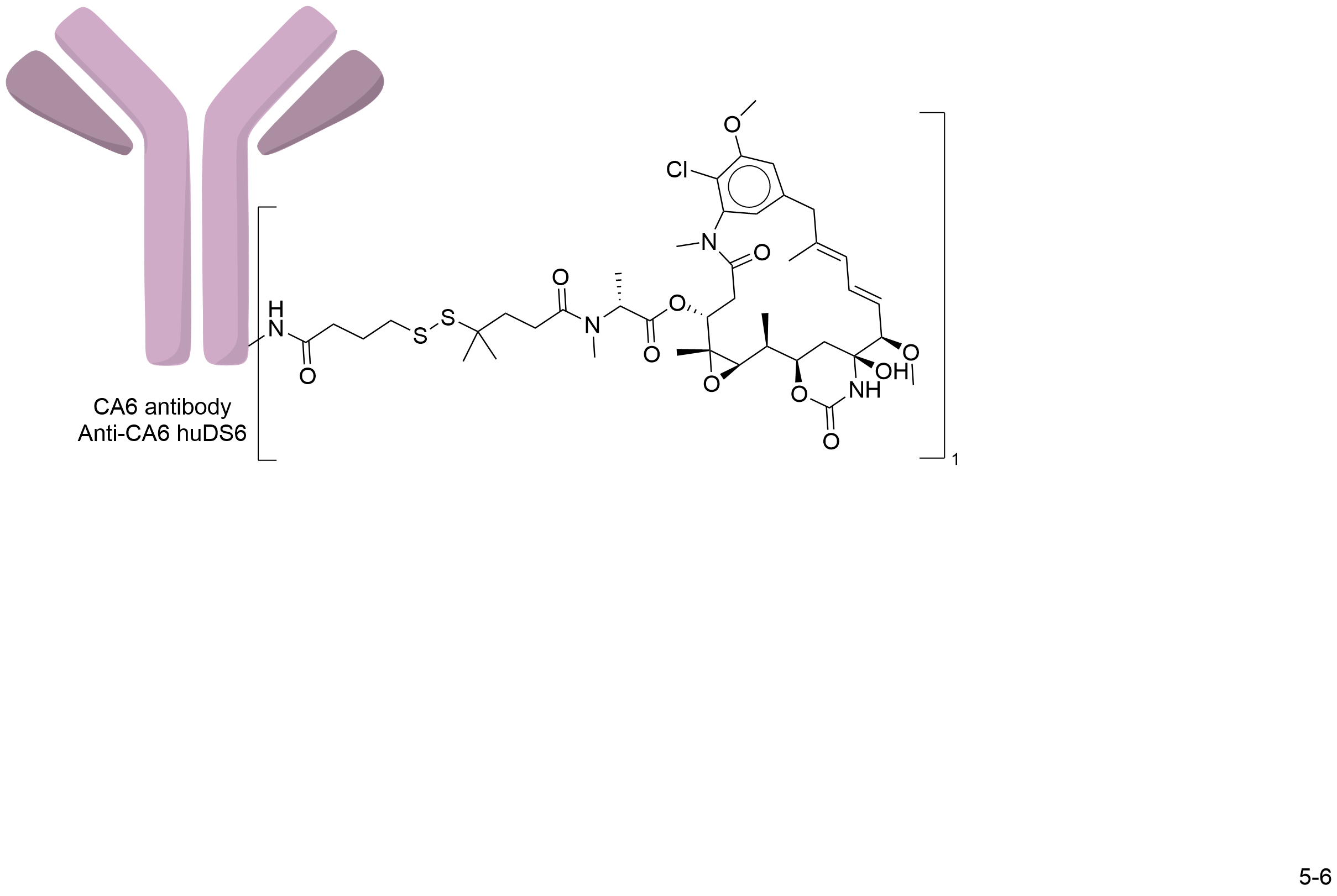
|
|||||
| Antibody Name |
Anti-CA6 mAb huDS6
|
Antibody Info | ||||
| Antigen Name |
Carbonic anhydrase 6 (CA6)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM4
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
N-succinimidyl 4-(2-pyridyldithio) butanoate (SPDB)
|
Linker Info | ||||
| Conjugate Type |
Random Lysines
|
|||||
| Combination Type |
ravtansine
|
|||||
| Puchem SID | ||||||
| DrugMap ID | ||||||
| TTD ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02984683 | Clinical Status | Phase 1 | ||
| Clinical Description | Open-label phase 2 study evaluating efficacy and safety of SAR566658 treatment in patients with CA6 positive metastatic triple negative breast cancer. | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT01156870 | Clinical Status | Phase 1 | ||
| Clinical Description | Dose escalation, safety and pharmacokinetic, first in man study, of SAR566658 administered as a single agent by intravenous infusion in adult patients with CA6-positive and refractory solid tumors. | ||||
References
