Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0GKOZH
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Mirvetuximab soravtansine
|
|||||
| Brand Name |
Elahere
|
|||||
| Synonyms |
IMGN853; M9346A-sulfo-SPDB-DM4; Anti-FOLR1 monoclonal antibody-maytansinoid conjugate; MIRV;HDM2002;IMGN-853;M9346-Asulfo-SPDB-DM4;M9346A-sSPDB-DM4;M9346A-sulfo-SPDB-DM4;Anti-FOLR1-monoclonal-antibody-maytansinoid-conjugate-IMGN-853;Mirvetuximab soravtansine-gynx
Click to Show/Hide
|
|||||
| Organization |
ImmunoGen, Inc.; Hangzhou Zhongmeihuadong Pharmaceutical Co., Ltd.
|
|||||
| Drug Status |
Approved (FDA): Nov, 2022
|
|||||
| Indication |
In total 5 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3.4
|
|||||
| Structure |
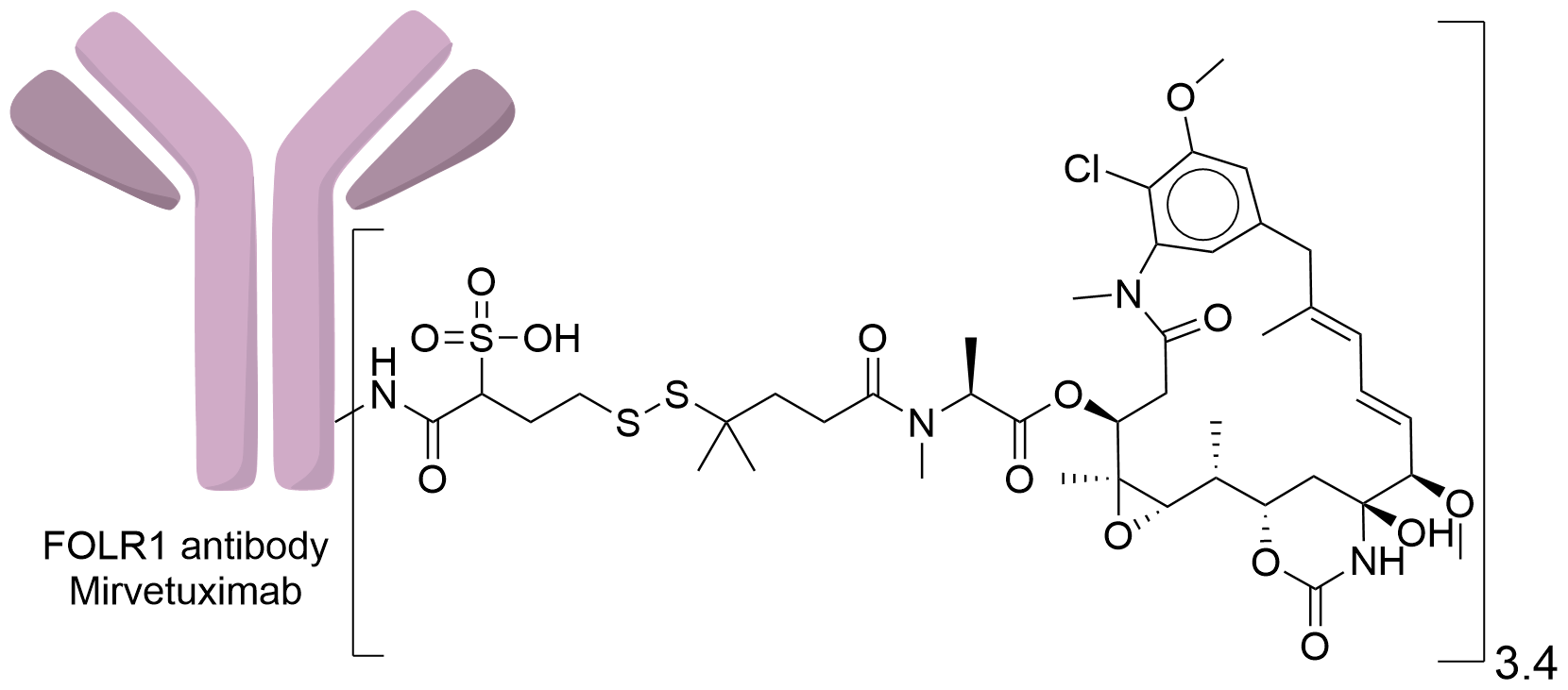
|
|||||
| Antibody Name |
Mirvetuximab
|
Antibody Info | ||||
| Antigen Name |
Folate receptor alpha (FOLR1)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM4
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Sulfo-SPDB
|
Linker Info | ||||
| Conjugate Type |
Random Lysines
|
|||||
| Combination Type |
Soravtansine
|
|||||
| Absorption |
For mirvetuximab soravtansine-gynx (6 mg/kg), mean Cmax was 137.3 (ug/mL) and mean AUC was 20.65 (mg x h/mL) following the first treatment cycle (21 days). For unconjugated DM4, mean Cmax was 4.11 (ng/mL) and mean AUC was 530 (ng x h/mL) following the first treatment cycle (21 days). For S-methyl-DM4, mean Cmax was 6.98 (ng/mL) and mean AUC was 1848 (ng x h/mL) following the first treatment cycle (21 days). The peak concentration of mirvetuximab soravtansine-gynx was observed near the end of intravenous infusion, while DM4 and S-methyl-DM4 concentrations peaked 2 and 3 days after administration.
|
|||||
| Distribution |
Mirvetuximab soravtansine-gynx has a steady-state volume of distribution of 2.63 L. Based on in vitro studies, the plasma protein binding of the mirvetuximab soravtansine-gynx component DM4 and its metabolite S-methyl DM4 is higher than 99%.
|
|||||
| Metabolism |
The monoclonal antibody portion of this drug is expected to be metabolized by catabolic pathways into small peptides. Unconjugated DM4 is reduced and S-methylated to form S-methyl-DM4. DM4 and S-methyl-DM4 are the main circulating metabolites of mirvetuximab soravtansine-gynx and correspond to approximately 0.4% and 1.4% of mirvetuximab soravtansine-gynx AUCs. Both DM4 and S-methyl-DM4 undergo metabolism by CYP3A4. After the first dose, the geometric mean terminal phase half-life of mirvetuximab soravtansine-gynx is 4.8 days. The geometric mean terminal phase half-lives of the unconjugated DM4 and its metabolite, S-methyl-DM4, are 2.8 and 5.0 days, respectively.
|
|||||
| Elimination |
Mirvetuximab soravtansine-gynx metabolites S-methyl DM4 and DM4-sulfo-SPDB-lysine were detected in urine within 24 hours. The total plasma clearance of mirvetuximab soravtansine-gynx is 18.9 mL/hour. The unconjugated DM4 has a total plasma clearance of 13.8 L/hour, while its metabolite, S-methyl-DM4, has a total plasma clearance of 4.3 L/hour.
|
|||||
| Toxicity |
Mirvetuximab soravtansine-gynx can cause severe ocular adverse reactions, including visual impairment, keratopathy (corneal disorders), dry eye, photophobia, eye pain, and uveitis. Ocular adverse reactions occurred in 61% of patients with ovarian cancer treated with ELAHERE.
|
|||||
| Special Approval(s) |
Priority review(FDA); Accelerated approval(FDA); Fast track(FDA); Orphan drug(FDA)
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| DrugMap ID | ||||||
| TTD ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Patient-derived Xenograft Model
Discovered Using Cell Line-derived Xenograft Model
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 22.00% | High FOLR1 expression (FOLR1+++) | ||
| Patients Enrolled |
Platinum-resistant epithelial ovarian cancer (EOC); Eligible patients with 1-3 prior lines of therapy and whose tumors were positive for FR expression.
|
||||
| Administration Dosage |
6 mg/kg Q3W.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02631876 | Clinical Status | Phase 3 | ||
| Clinical Description | FORWARD I: A randomized, open label phase 3 study to evaluate the safety and efficacy of mirvetuximab soravtansine (IMGN853) versus investigator's choice of chemotherapy in women with folate receptor alpha positive advanced epithelial ovarian cancer, primary peritoneal cancer or fallopian tube cancer. | ||||
| Primary Endpoint |
For the ITT population, Kaplan-Meier estimates showed no significant difference (HR, 0.98; 95% Cl, 0.73-1.31; P=0.897) in progression-free survival (PFS) between groups Median PFS was 4.10 and 4.40 months for MIRV and IC chemotherapy, respectively. In the prespecified high FR subgroup, PFS was longer in patients in the MIRV group compared with IC chemotherapy (median, 4.80 months versus 3.30 months; HR, 0.69, 95% CI, 0.48 to 1.00; P = 0.049). Based on the Hochberg procedure used in the statistical analysis plan for the study, this P value did not meet statistical significanceBoth in the ITT group and high FR subgroup, OS were not significantly different.
Click to Show/Hide
|
||||
| Other Endpoint |
Mirvetuximab soravtansine vs IC chemotherapy in the ITT group: ORR=22.00% vs 12.00%, P=0.015; cancer antigen-125 (CA-125) responses=51.00% vs 27.00%, P<0001; median PFS2 duration (time from randomization to second disease progression or death)=10.00 vs 8.40 months, HR=0.64, 95% Cl, 0.49-0.84, P<0.001); PRO in the EORTC QLQ-OV28 Abdominal/GI Symptom Subscale=32.00% vs 14.00%, P=0.016; Mirvetuximab soravtansine vs IC chemotherapy in the high FR subset, ORR=24.00% vs 10.00%, P=0.014; cancer antigen-125 (CA-125) responses=53.00% vs 25.00%, P=0.001; median PFS2 duration=10.10 vs 8.40 months, HR=0.56, 95% Cl, 0.39-0.79, P<0.001); PRO in the EORTC QLQ-OV28 Abdominal/GI Symptom Subscale=27.00% vs 13.00%, P=0.143.
Click to Show/Hide
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 31.73% | High FOLR1 expression (FOLR1+++) | ||
| Patients Enrolled |
Platinum-Resistant, Advanced High-Grade Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancers With High Folate Receptor-Alpha Expression.
|
||||
| Administration Dosage |
6 mg/kg Q3W.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT04296890 | Clinical Status | Phase 3 | ||
| Clinical Description | SORAYA: A Phase 3, single arm study of mirvetuximab soravtansine in platinum-resistant, advanced high-grade epithelial ovarian, primary peritoneal, or fallopian tube cancers with high folate receptor-alpha expression. | ||||
| Primary Endpoint |
Confirmed Overall Response Rate=31.73% [(N=104, 95% Cl, 22.90-41.60), Complete response rate=4.80%, Partial response rate=26.90%].
|
||||
| Other Endpoint |
Median duration of response=6.90 months (N=33, 95% Cl 5.60-9.70).
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 39.00% | Moderate FOLR1 expression (FOLR1++) | ||
| Patients Enrolled |
Platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer.
|
||||
| Administration Dosage |
6 mg/kg (adjusted ideal body weight) once every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02606305 | Clinical Status | Phase 1b/2 | ||
| Clinical Description | A phase 1b/2 study to evaluate the safety, tolerability and pharmacokinetics of mirvetuximab soravtansine (IMGN853) in combination with bevacizumab, carboplatin, pegylated liposomal doxorubicin, pembrolizumab, or bevacizumab + carboplatin, in adults with folate receptor alpha positive advanced epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer. | ||||
| Primary Endpoint |
For AURELIA-type patients (N=16, bevacizumab-naive and 1-2 prior lines of therapy, plus medium/high FRa expression), confirmed ORR=56.00%, median duration of response (mDOR)=12.0 months, median progression-free survival (mPFS) = 9.9 months.
|
||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.40% (Day 30) | Moderate ROR1 expression (ROR1++) | ||
| Method Description |
In vivoexperiments comparing the antitumor activity of IMGN853 versus ADC isotype control, PBS and M9346A were conducted using both high grade endometrioid/clear cell xenografts as well as a uterine serous tumor PDX model (BIO (K)1).all treatments were given twice, one week apart by retro-orbital injection at a concentration of 5 mg/kg.
|
||||
| In Vivo Model | Uterine serous carcinomas PDX model (PDX: BIO(K)1) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 25) | Moderate ROR1 expression (ROR1++) | ||
| Method Description |
In vivoexperiments comparing the antitumor activity of IMGN853 versus ADC isotype control, PBS and M9346A were conducted using both high grade endometrioid/clear cell xenografts as well as a uterine serous tumor PDX model (BIO (K)1).all treatments were given twice, one week apart by retro-orbital injection at a concentration of 5 mg/kg.
|
||||
| In Vivo Model | Uterine serous carcinomas PDX model (PDX: END(K)265) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 33.00% (Day 5) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OV-90 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 25 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OV-90 CDX model | ||||
| In Vitro Model | Ovarian adenocarcinoma | OV-90 cells | CVCL_3768 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.00% (Day 6) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of IGROV-1 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 25 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | IGROV-1 CDX model | ||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 64.00% (Day 26) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OV-90 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 50 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OV-90 CDX model | ||||
| In Vitro Model | Ovarian adenocarcinoma | OV-90 cells | CVCL_3768 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 82.00% (Day 40) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OV-90 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 100 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OV-90 CDX model | ||||
| In Vitro Model | Ovarian adenocarcinoma | OV-90 cells | CVCL_3768 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 82.00% (Day 21) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OVCAR-3 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 25 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 93.00% (Day 32) | High FOLR1 expression (FOLR1+++; 1,300,000 FOLR1 molecules/cell) | ||
| Method Description |
Animals with established tumors of about 130 mm3 were treated with intravenous single injection of the M9346A-DM conjugates at 2.5±0.2 mg/kg, equivalent to 51±3 ug conjugated maytansinoid per kg The conjugates were injected on day 7 after cell inoculation.
|
||||
| In Vivo Model | Ovarian carcinoma Igrov-1 CDX model | ||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.00% (Day 41) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of IGROV-1 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 50 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | IGROV-1 CDX model | ||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.00% (Day 67) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of IGROV-1 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 100 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | IGROV-1 CDX model | ||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 99.07% (Day 30) | High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Animals with established tumors of about 130 mm3 were treated with intravenous single injection of the M9346A-DM conjugates at 2.5±0.2 mg/kg, equivalent to 51±3 ug conjugated maytansinoid per kg The conjugates were injected on day 20 after cell inoculation.
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 75) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OVCAR-3 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 50 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 120) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OVCAR-3 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 100 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.15±0.08 nM
|
|||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.20±0.10 nM
|
|||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Gestational choriocarcinoma | JEG-3 cells | CVCL_0363 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00±0.40 nM
|
|||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.20 ug/mL | Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
Cell cytotoxicity was tested with IMGN853, ADC isotype control and M9346A in pure and mixed endometrial endometrioid cell lines harboring high FOLR1 expression.
|
||||
| In Vitro Model | Endometrial undifferentiated carcinoma | END-2 cells | CVCL_B5KE | ||
| Experiment 5 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 74.60 ng/mL | Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
Cell cytotoxicity was tested with IMGN853, ADC isotype control and M9346A in pure and mixed endometrial endometrioid cell lines harboring high FOLR1 expression.
|
||||
| In Vitro Model | Endometrial undifferentiated carcinoma | END-1 cells | CVCL_B5JE | ||
References
