Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0MOENC
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
CNTO95-SPDB-DM4
|
|||||
| Synonyms |
CNTO95 SPDB DM4
Click to Show/Hide
|
|||||
| Organization |
Sanofi SA
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 3 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3-4
|
|||||
| Structure |
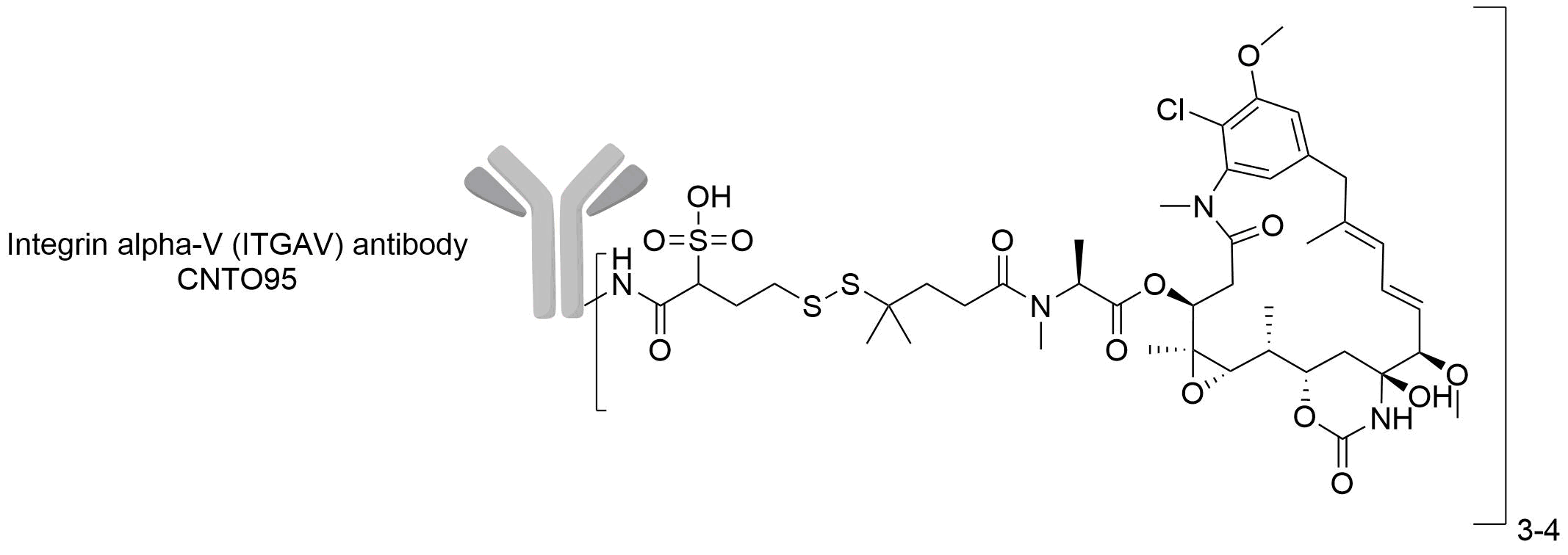
|
|||||
| Antibody Name |
CNTO95
|
Antibody Info | ||||
| Antigen Name |
Integrin alpha-V (ITGAV)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM4
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
N-succinimidyl 4-(2-pyridyldithio) butanoate (SPDB)
|
Linker Info | ||||
| Conjugate Type |
Random conjugation through nucleophilic lysines.
|
|||||
| Combination Type |
Ravtansine
|
|||||
General Information of The Activity Data Related to This ADC
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 0.24 ug/mL | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were seeded into white 96-well tissue culture plates (5000 cells/well)in culture medium and incubated for 16 hrs. Serial dilutions of immunoconjugates were added toeach appropraite wells (0-20 ug/ml). Tissue culture plates were incubated at 37°C for 96 hrs.
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 0.30 ug/mL | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were seeded into white 96-well tissue culture plates (5000 cells/well)in culture medium and incubated for 16 hrs. Serial dilutions of immunoconjugates were added toeach appropraite wells (0-20 ug/ml). Tissue culture plates were incubated at 37°C for 96 hrs.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 0.21 mg/mL | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 0.27 mg/mL | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | A2780 cells | CVCL_0134 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 0.34 mg/mL | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
