Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0QTTWI
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Praluzatamab ravtansine
|
|||||
| Synonyms |
Anti-CD166-DM4-CX-2009; Anti-cd166 probody-drug conjugate cx-2009; CD166 Probody Drug Conjugate; CD166 probody drug conjugate; CX-2009; Praluzatamab ravtansine
Click to Show/Hide
|
|||||
| Organization |
ImmunoGen, Inc.; CytomX Therapeutics, Inc.
|
|||||
| Drug Status |
Phase 2
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3.5
|
|||||
| Structure |
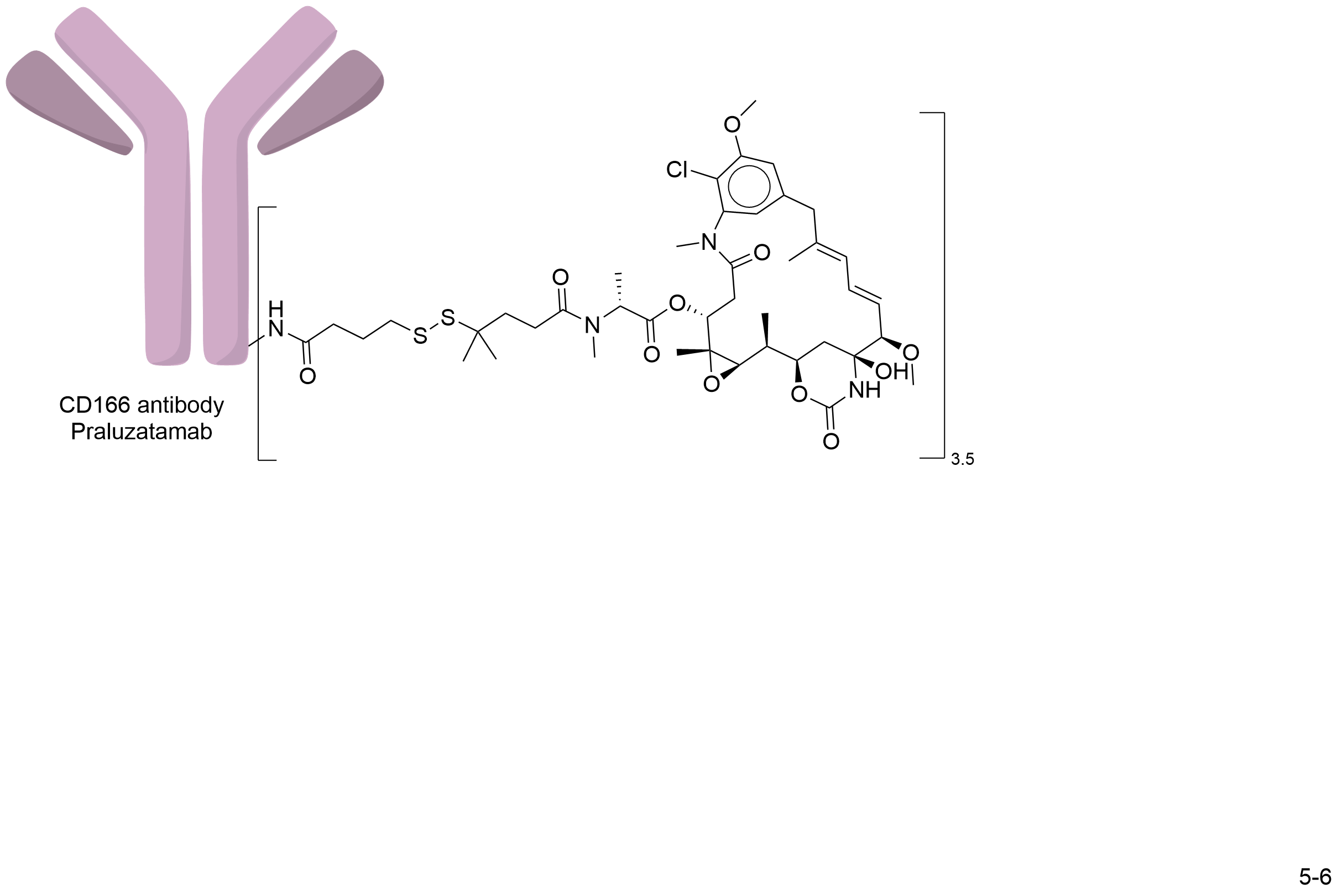
|
|||||
| Antibody Name |
Praluzatamab
|
Antibody Info | ||||
| Antigen Name |
CD166 antigen (ALCAM)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM4
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
N-succinimidyl 4-(2-pyridyldithio) butanoate (SPDB)
|
Linker Info | ||||
| Conjugate Type |
Random Lysines
|
|||||
| Combination Type |
ravtansine
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Partial Response (PR) |
9.00%
|
|||
| Patients Enrolled |
Eastern Cooperative Oncology Group (ECOG) 0, 1, metastatic or locally advanced unresectable solid tumors with progressive disease (PD) after standard treatment or known intolerance to available treatment, based on the predicted prevalence of CD166 expression, were breast cancer, castration-resistant prostate cancer, nonsmall cell lung cancer (NSCLC), epithelial ovarian cancer, head and neck squamous cell cancer (HNSCC), cholangiocarcinoma, and endometrial carcinoma.
Click to Show/Hide
|
||||
| Administration Dosage |
Scalating doses every 3 weeks (0.25-10 mg/kg) or every 2 weeks (4-6 mg/kg), IV.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03149549 | Clinical Status | Phase 1/2 | ||
| Clinical Description | A phase 1-2, open-label, dose-finding, proof of concept, first-in-human study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of CX-2009 in adults with metastatic or locally advanced unresectable solid tumors (PROCLAIM-CX-2009). | ||||
| Primary Endpoint |
Median number of prior therapies was 5. On the basis of tolerability, the RP2D was 7 mg/kg every 3 weeks. Tumor regressions were observed at doses 4 mg/kg.
|
||||
References
