Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0ELYWP
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Tusamitamab ravtansine
|
|||||
| Synonyms |
AR408701; IBI-126; Maytansin-loaded anti-CEACAM5 mAb; SAR 408701; SAR-408701; SAR408701; Tusamitamab ravtansine
Click to Show/Hide
|
|||||
| Organization |
ImmunoGen, Inc.; Sanofi; Innovent Biologics, Inc.
|
|||||
| Drug Status |
Phase 3
|
|||||
| Indication |
In total 5 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3 to 4
|
|||||
| Structure |
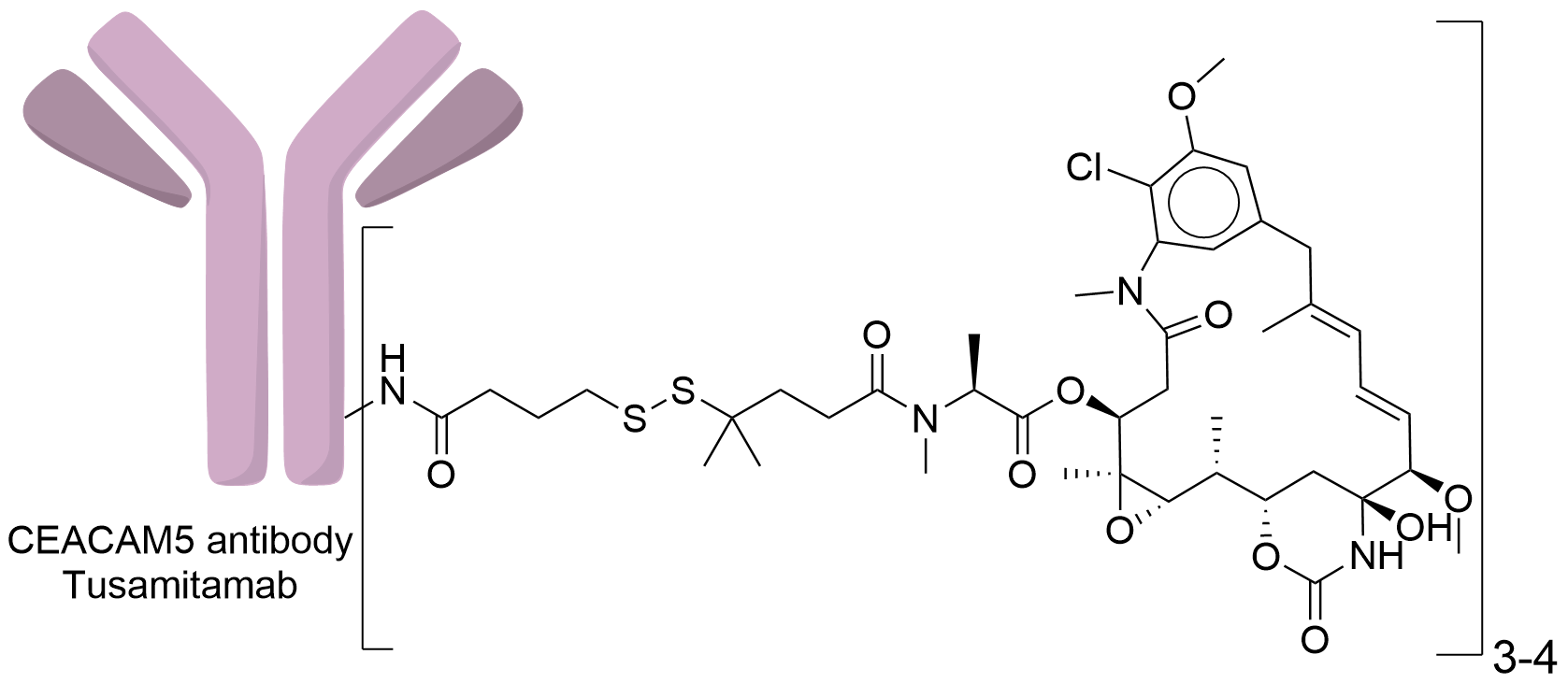
|
|||||
| Antibody Name |
Tusamitamab
|
Antibody Info | ||||
| Antigen Name |
Carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM4
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
N-succinimidyl 4-(2-pyridyldithio) butanoate (SPDB)
|
Linker Info | ||||
| Conjugate Type |
Random Lysines
|
|||||
| Combination Type |
ravtansine
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Patient-derived Xenograft Model
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 40.00% | Low CEACAM5 expression (CEACAM5+; 299,300 sites/cell) | ||
| Patients Enrolled |
Patients with advanced/metastatic nonsquamous non-small cell lung cancer (NSQ NSCLC) with CEACAM5 intensity of 2+ in 1% of tumor cells by immunohistochemistry.
|
||||
| Administration Dosage |
IV Q3W at 150 or 170 mg/m2.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT04524689 | Clinical Status | Phase 2 | ||
| Clinical Description | Open-label, phase 2 study of tusamitamab ravtansine (SAR408701) combined with pembrolizumab and tusamitamab ravtansine (SAR408701) combined with pembrolizumab and platinum-based chemotherapy with or without pemetrexed in patients with CEACAM5 positive expression advanced/metastatic non-squamous non-small-cell lung cancer (nsq NSCLC). | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 20.30% (CEACAM5 High-expression), 7.10% (CEACAM5 Moderate-expression), 41.70% (high cCEA), 8.10% (Low cCEA) | Moderate CEACAM5 expression (CEACAM5++; 1,615,700 sites/cell) | ||
| Patients Enrolled |
Enrolled 2 cohorts of patients with IHC CEACAM5 membrane expression at 2+ intensity: in 50% of tumor cells (high expressors, HEs, n = 64); and in 1% to <50% of tumor cells (moderate expressors, MEs, n = 28).
|
||||
| Administration Dosage |
100 mg/m2 IV every 2 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02187848 | Clinical Status | Phase 1 | ||
| Clinical Description | A first-in-human study for the evaluation of the safety, pharmacokinetics and antitumor activity of SAR408701 in patients with advanced solid tumors. | ||||
| Primary Endpoint |
The primary endpoint was the incidence of DLTs occurring during the first two cycles (4 weeks) of study drug administration. DLTs (reversible grade 3 microcystic keratopathy) occurred in three of eight patients treated with tusamitamab ravtansine 12.00 mg/m2 and in two of three patients treated with 15.00 mg/m2. The maximum tolerated dose was identified as 10.00 mg/m2.
Click to Show/Hide
|
||||
| Other Endpoint |
Three patients (9.68%) had objective responses [all confirmed partial responses (PRs) with durations of 2.60, 6.10, and 4.00 months]; 11 patients (35.48%) had stable disease, and 13 patients (41.94%) had progressive disease. Objective responses were achieved in two of six patients (33.33%) at a DL of 10.0 mg/m2, and in one of nine patients (11.11%) at 12.0 mg/m2 with maximum reduction in RECIST target lesions of 32.30%-51.20%.
Click to Show/Hide
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05703555 | Clinical Status | Phase 2 | ||
| Clinical Description | Intrusion: unraveling the intratumoral PK/PD relation for SAR408701. | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05245071 | Clinical Status | Phase 2 | ||
| Clinical Description | Open-label, phase 2 study, evaluating the efficacy and safety of tusamitamab ravtansine in non-squamous non-small-cell lung cancer (nsq NSCLC) participants with negative or moderate CEACAM5 expression tumors and high circulating CEA. | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05071053 | Clinical Status | Phase 2 | ||
| Clinical Description | Open-label study of tusamitamab ravtansine (SAR408701) in combination with ramucirumab in participants previously treated for advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma with CEACAM5-positive tumors. | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [6] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04659603 | Clinical Status | Phase 2 | ||
| Clinical Description | Open-label, multi-cohort, phase 2 trial, evaluating the efficacy and safety of tusamitamab ravtansine (SAR408701) monotherapy and in combination in patients with CEACAM5-positive advanced solid tumors. | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [7] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05429762 | Clinical Status | Phase 1 | ||
| Clinical Description | Open-label study evaluating the effect of tusamitamab ravtansine on the QTC interval in participants with metastatic solid tumors. | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [8] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04154956 | Clinical Status | Phase 1 | ||
| Clinical Description | Randomized, open-label, phase 3 study of SAR408701 versus docetaxel in previously treated, metastatic nonsquamous, non-small-cell lung cancer patients with CEACAM5-positive tumors. | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [9] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03324113 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 study to evaluate safety and pharmacokinetics of SAR408701 administered intravenously as monotherapy in japanese patients with advanced malignant solid tumors. | ||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 0.00% (Day 28) | Moderate CEACAM5 expression (CEACAM5++; IHC 2+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a lung adenocarcinoma patient with IHC 2+, with a single intravenous administration at 2.5 mg/kg.
|
||||
| In Vivo Model | Lung adenocarcinoma PDX model (PDX: LUN-NIC-014) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 6.40% (Day 28) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, with a single intravenous administration at 2.5 mg/kg.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-002C/M) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 27.10% (Day 28) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a stomach adenocarcinoma patient with IHC 3+, with a single intravenous administration at 2.5 mg/kg.
|
||||
| In Vivo Model | Stomach adenocarcinoma PDX model (PDX: STO-IND-0007) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 32.90% (Day 28) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, with a single intravenous administration at 5 mg/kg.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-002C/M) | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 41.50% (Day 17) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a stomach adenocarcinoma patient with IHC 3+, with a single intravenous administration at 2.5 mg/kg.
|
||||
| In Vivo Model | Stomach adenocarcinoma PDX model (PDX: SA-STO-0014) | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 55.00% (Day 28) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a stomach adenocarcinoma patient with IHC 3+, twice a week with a single intravenous administration at 2.5 mg/kg for a total of 4 weeks.
|
||||
| In Vivo Model | Stomach adenocarcinoma PDX model (PDX: STO-IND-0007) | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 57.20% (Day 17) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a stomach adenocarcinoma patient with IHC 3+, with a single intravenous administration at 5 mg/kg.
|
||||
| In Vivo Model | Stomach adenocarcinoma PDX model (PDX: SA-STO-0014) | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 60.30% (Day 28) | Moderate CEACAM5 expression (CEACAM5++; IHC 2+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a lung adenocarcinoma patient with IHC 2+, with a single intravenous administration at 2.5 mg/kg.
|
||||
| In Vivo Model | Lung adenocarcinoma PDX model (PDX: LUN-NIC-0083) | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 63.90% (Day 28) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, with a single intravenous administration at 2.5 mg/kg.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-0034P) | ||||
| Experiment 10 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 66.20% (Day 28) | Moderate CEACAM5 expression (CEACAM5++; IHC 2+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a lung adenocarcinoma patient with IHC 2+, with a single intravenous administration at 5 mg/kg.
|
||||
| In Vivo Model | Lung adenocarcinoma PDX model (PDX: LUN-NIC-0083) | ||||
| Experiment 11 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 69.70% (Day 28) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a stomach adenocarcinoma patient with IHC 3+, with a single intravenous administration at 5 mg/kg.
|
||||
| In Vivo Model | Stomach adenocarcinoma PDX model (PDX: STO-IND-0007) | ||||
| Experiment 12 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 69.80% (Day 28) | Moderate CEACAM5 expression (CEACAM5++; IHC 2+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a lung adenocarcinoma patient with IHC 2+, with a single intravenous administration at 10 mg/kg.
|
||||
| In Vivo Model | Lung adenocarcinoma PDX model (PDX: LUN-NIC-0083) | ||||
| Experiment 13 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 70.20% (Day 28) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a stomach adenocarcinoma patient with IHC 3+, with a single intravenous administration at 5 mg/kg.
|
||||
| In Vivo Model | Stomach adenocarcinoma PDX model (PDX: STO-IND-0007) | ||||
| Experiment 14 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 71.80% (Day 28) | Moderate CEACAM5 expression (CEACAM5++; IHC 2+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a lung adenocarcinoma patient with IHC 2+, with a single intravenous administration at 5 mg/kg.
|
||||
| In Vivo Model | Lung adenocarcinoma PDX model (PDX: LUN-NIC-014) | ||||
| Experiment 15 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 76.50% (Day 28) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a stomach adenocarcinoma patient with IHC 3+, once a week with a single intravenous administration at 5 mg/kg for a total of 4 weeks.
|
||||
| In Vivo Model | Stomach adenocarcinoma PDX model (PDX: STO-IND-0007) | ||||
| Experiment 16 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 84.70% (Day 28) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, with a single intravenous administration at 5 mg/kg.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-0034P) | ||||
| Experiment 17 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 90.20% (Day 28) | Moderate CEACAM5 expression (CEACAM5++; IHC 2+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a lung adenocarcinoma patient with IHC 2+, with a single intravenous administration at 10 mg/kg.
|
||||
| In Vivo Model | Lung adenocarcinoma PDX model (PDX: LUN-NIC-014) | ||||
| Experiment 18 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 90.30% (Day 28) | Moderate CEACAM5 expression (CEACAM5++; IHC 2+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a lung adenocarcinoma patient with IHC 2+, twice a week with a single intravenous administration at 2.5 mg/kg for a total of 4 weeks.
|
||||
| In Vivo Model | Lung adenocarcinoma PDX model (PDX: LUN-NIC-014) | ||||
| Experiment 19 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 91.40% (Day 28) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, with a single intravenous administration at 10 mg/kg.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-002C/M) | ||||
| Experiment 20 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 92.40% (Day 28) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a stomach adenocarcinoma patient with IHC 3+, with a single intravenous administration at 10 mg/kg.
|
||||
| In Vivo Model | Stomach adenocarcinoma PDX model (PDX: STO-IND-0007) | ||||
| Experiment 21 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 92.80% (Day 28) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, with a single intravenous administration at 10 mg/kg.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-0034P) | ||||
| Experiment 22 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 92.80% (Day 28) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, with a single intravenous administration at 5 mg/kg.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-0034P) | ||||
| Experiment 23 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 93.30% (Day 17) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a stomach adenocarcinoma patient with IHC 3+, with a single intravenous administration at 10 mg/kg.
|
||||
| In Vivo Model | Stomach adenocarcinoma PDX model (PDX: SA-STO-0014) | ||||
| Experiment 24 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 93.50% (Day 28) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, with a single intravenous administration at 5 mg/kg.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-002C/M) | ||||
| Experiment 25 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 97.00% (Day 28) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, once a week with a single intravenous administration at 5 mg/kg for a total of 4 weeks.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-0034P) | ||||
| Experiment 26 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 99.40% (Day 28) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, once a week with a single intravenous administration at 5 mg/kg for a total of 4 weeks.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-002C/M) | ||||
| Experiment 27 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 99.90% (Day 28) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, twice a week with a single intravenous administration at 2.5 mg/kg for a total of 4 weeks.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-002C/M) | ||||
| Experiment 28 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 100.00% (Day 28) | High CEACAM5 expression (CEACAM5+++; IHC 3+) | ||
| Method Description |
Tusamitamab ravtansine induces efficient tumor cell killing in PDX models of a colon adenocarcinoma patient with IHC 3+, twice a week with a single intravenous administration at 2.5 mg/kg for a total of 4 weeks.
|
||||
| In Vivo Model | Colon adenocarcinoma PDX model (PDX: CR-IGR-0034P) | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.20±0.04 nM | Low CEACAM5 expression (CEACAM5+; 498,900 sites/cell) | ||
| Method Description |
The inhibitory activity of SAR408377 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated incubated overnight.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.38±0.07 nM
|
|||
| Method Description |
The inhibitory activity of SAR408377 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated incubated overnight.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.08±0.17 nM
|
|||
| Method Description |
The inhibitory activity of SAR408377 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated incubated overnight.
|
||||
| In Vitro Model | Gastric adenocarcinoma | MKN45 cells | CVCL_0434 | ||
References
