Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0AWUIJ
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Indatuximab ravtansine
|
|||||
| Synonyms |
Anti-myeloma monoclonal antibody-DM4 immunoconjugate BT-062; BT-062; Indatuximab ravtansine; Maytansinoid-conjugated anti-myeloma monoclonal antibody BT-062; nBT-062
Click to Show/Hide
|
|||||
| Organization |
Biotest Pharma GmbH; Biotest AG
|
|||||
| Drug Status |
Phase 1/2 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3 to 4
|
|||||
| Structure |
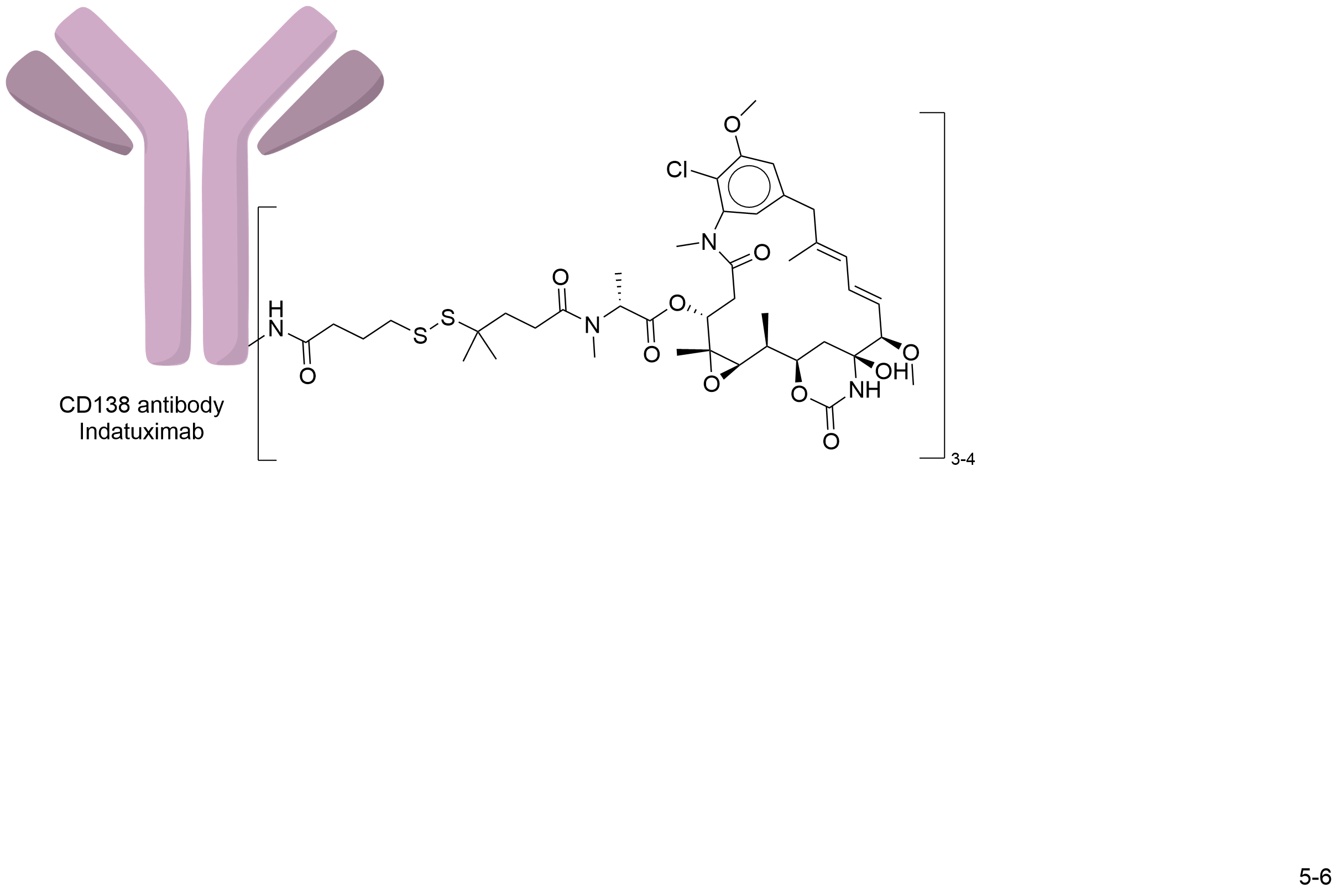
|
|||||
| Antibody Name |
Indatuximab
|
Antibody Info | ||||
| Antigen Name |
Syndecan-1 (SDC1)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM4
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
N-succinimidyl 4-(2-pyridyldithio) butanoate (SPDB)
|
Linker Info | ||||
| Conjugate Type |
Random Lysines
|
|||||
| Combination Type |
ravtansine
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| TTD ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Patient-derived Xenograft Model
Discovered Using Cell Line-derived Xenograft Model
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
3.20% (on the single-dose regimen)
5.90% (the multi-dose regimen) |
|||
| Patients Enrolled |
Relapsed and/or refractory multiple myeloma (MM) previously treated with an immunomodulatory drug and a proteasome inhibitor.
|
||||
| Administration Dosage |
In the first-in-human study, indatuximab ravtansine (10, 20, 40, 80, 120, 160, 200 mg/m2) was administered to 32 patients on day 1 of each 21-day cycle. The MTD was 160 mg/m2. In the phase I/IIa study, indatuximab ravtansine (40, 50, 65, 80, 100, 120, 140, 160 mg/m2) was administered to 35 patients on days 1, 8, and 15 of each 28-day cycle, and the MTD/recommended phase II dose was 140 mg/m2.
Click to Show/Hide
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT00723359 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 dose escalation study to evaluate maximum tolerated dose (MTD), pharmacokinetics (PK), and safety of BT062 in subjects with relapsed or relapsed/refractory multiple myeloma. | ||||
| Primary Endpoint |
The 160 mg/m2 dose was therefore defined as MTD for the Single-dose Regimen.
|
||||
| Other Endpoint |
Most (88.00%) adverse events were grade 1 or 2, the most common being diarrhea and fatigue. There was rapid clearance of indatuximab ravtansine and no relevant accumulation.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Patients Enrolled |
Relapsed or refractory multiple myeloma, and ECOG performance status or Zubrod score of 2 or below.
|
||||
| Administration Dosage |
Intravenously on days 1, 8, and 15 of each 28-day cycle in escalating dose levels of 80 mg/m2, 100 mg/m2, and 120 mg/m2, with lenalidomide (25 mg; days 1 to 21 every 28 days orally) and dexamethasone (20-40 mg; days 1, 8, 15, and 22 every 28 days) (phase 1).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01638936 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/2a multi-dose escalation study of BT062 in combination with lenalidomide or pomalidomide and dexamethasone in subjects with relapsed or relapsed/refractory multiple myeloma. | ||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 39) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 1 mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF1384) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 2 mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF1384) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 39) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 4 mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF1384) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 25.00% (Day 38) | High CD138 expression (CD138+++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 1 mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF401) | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 55.00% (Day 38) | High CD138 expression (CD138+++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 2 mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF401) | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 55.00% (Day 39) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine combination docetaxel against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated docetaxel with 10 mg/kg and treated indatuximab ravtansine with 2 mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF1384) | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 70.00% (Day 39) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 8mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF1384) | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.50% (Day 38) | High CD138 expression (CD138+++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 4 mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF401) | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.80% (Day 38) | High CD138 expression (CD138+++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 8mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF401) | ||||
| Experiment 10 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 38) | High CD138 expression (CD138+++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine combination docetaxel against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated docetaxel with 10 mg/kg and treated indatuximab ravtansine with 2 mg/kg.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: MAXF401) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 38.86% (Day 14) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine combination lenalidomide against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 5.3 mg/kg body weight for 14 days.
|
||||
| In Vivo Model | Multiple myeloma CDX model | ||||
| In Vitro Model | Multiple myeloma | Multiple myeloma cells | Homo sapiens | ||
| Experiment 2 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 40.42% (Day 17) | High CD138 expression (CD138+++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 2 mg/kg/day.
|
||||
| In Vitro Model | Plasma cell myeloma | MMXF L363 cells | Homo sapiens | ||
| Experiment 3 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 50.00% (Day 14) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 5.3 mg/kg body weight for 14 days.
|
||||
| In Vivo Model | Multiple myeloma CDX model | ||||
| In Vitro Model | Multiple myeloma | Multiple myeloma cells | Homo sapiens | ||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 55.69% (Day 14) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine combination lenalidomide against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 10.6 mg/kg body weight for 14 days.
|
||||
| In Vivo Model | Multiple myeloma CDX model | ||||
| In Vitro Model | Multiple myeloma | Multiple myeloma cells | Homo sapiens | ||
| Experiment 5 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 59.90% (Day 14) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 10.6 mg/kg body weight for 14 days.
|
||||
| In Vivo Model | Multiple myeloma CDX model | ||||
| In Vitro Model | Multiple myeloma | Multiple myeloma cells | Homo sapiens | ||
| Experiment 6 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 65.34% (Day 14) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 21.2 mg/kg body weight for 14 days.
|
||||
| In Vivo Model | Multiple myeloma CDX model | ||||
| In Vitro Model | Multiple myeloma | Multiple myeloma cells | Homo sapiens | ||
| Experiment 7 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.26% (Day 17) | High CD138 expression (CD138+++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 4 mg/kg/day.
|
||||
| In Vitro Model | Plasma cell myeloma | MMXF L363 cells | Homo sapiens | ||
| Experiment 8 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.27% (Day 17) | High CD138 expression (CD138+++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine+lenalidomide (Len; 20 mg/kg/day) and dexamethasone (1.25 mg/kg/day) against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated IR with 2 mg/kg/day.
|
||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 79.20% (Day 14) | Moderate CD138 expression (CD138++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine combination lenalidomide against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with 21.2 mg/kg body weight for 14 days.
|
||||
| In Vivo Model | Multiple myeloma CDX model | ||||
| In Vitro Model | Multiple myeloma | Multiple myeloma cells | Homo sapiens | ||
| Experiment 10 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 92.05% (Day 17) | High CD138 expression (CD138+++) | ||
| Method Description |
The inhibitory activity of indatuximab ravtansine+lenalidomide (Len; 20 mg/kg/day) and dexamethasone (1.25 mg/kg/day) against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated IR with 4 mg/kg/day.
|
||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
References
