Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0CRDKL
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
HKT-288
|
|||||
| Synonyms |
HKT 288; HKT-288; HKT28 8, NOV-13; HKT288; NOV-13
Click to Show/Hide
|
|||||
| Organization |
Novartis Pharma AG; Novartis AG
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 4 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
Undisclosed
|
|||||
| Structure |
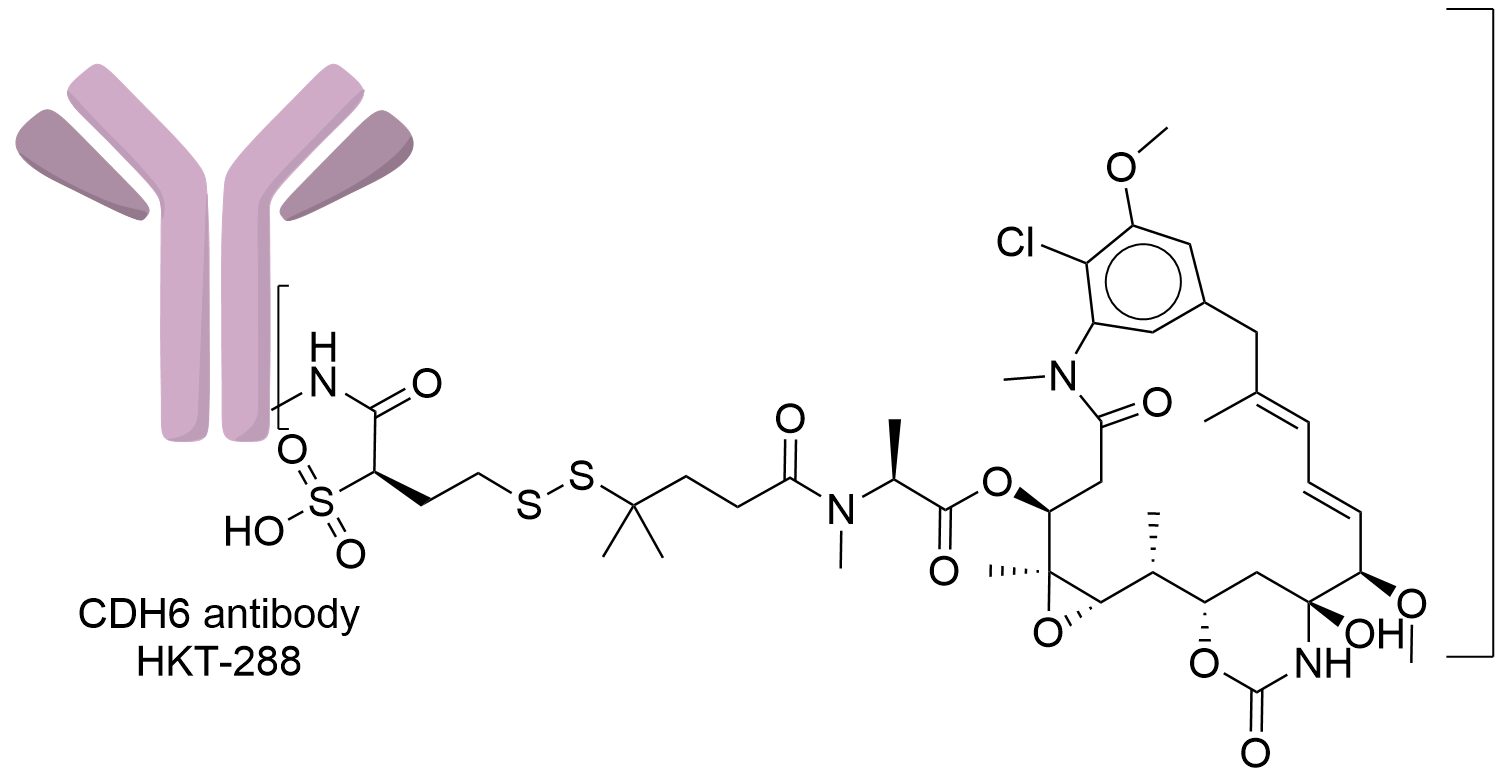
|
|||||
| Antibody Name |
Fully human Anti-CDH6 HKT-288 mAb
|
Antibody Info | ||||
| Antigen Name |
Cadherin-6 (CDH6)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM4
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Sulfo-SPDB
|
Linker Info | ||||
| Conjugate Type |
Random Lysines
|
|||||
| Puchem SID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Cell Line-derived Xenograft Model
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Patients Enrolled |
Advanced (metastatic or locally advanced) serous epithelial ovarian, serous fallopian tubal or serous primary peritoneal cancer or advanced clear cell or papillary renal cell carcinoma (RCC), who had received or were intolerant to all therapies known to confer clinical benefit for their disease and Eastern Cooperative Oncology Group (ECOG) performance status 2.
Click to Show/Hide
|
||||
| Administration Dosage |
HKT288 was administered intravenously (IV) every 3 weeks until patients experienced unacceptable toxicity or progressive disease (PD). The starting dose of 0.30 mg/kg was determined based on the highest nonseverely toxic dose in monkeys, which was 2 mg/kg IV weekly.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02947152 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, multicenter, open-label dose escalation and expansion study of HKT288, administered intravenously in adult patients with advanced solid tumors, including epithelial ovarian cancer and renal cell carcinoma. | ||||
| Primary Endpoint |
The best overall response on the 0.30 mg/kg cohort in patients with measurable disease was RECIST v1.1 stable disease in 3 patients and PD in 2 patients.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Patients Enrolled |
Patients with advanced solid tumors, including epithelial ovarian cancer and renal cell carcinoma.
|
||||
| Administration Dosage |
Cadherin-6-targeting ADC iv at dose of 0.30, 0.75 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02947152 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, multicenter, open-label dose escalation and expansion study of HKT288, administered intravenously in adult patients with advanced solid tumors, including epithelial ovarian cancer and renal cell carcinoma. | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.00% (Day 45) | High CDH6 expression (CDH6+++) | ||
| Method Description |
CDH6-sulfo-DM4 induces efficient tumor cell killing in cell PDX models with CDH6 expression.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 45) | High CDH6 expression (CDH6+++) | ||
| Method Description |
CDH6-sulfo-DM4 induces efficient tumor cell killing in cell PDX models with CDH6 expression.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
References
