Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0XCWMD
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Cantuzumab ravtansine
|
|||||
| Synonyms |
C-242 DM4; C242 DM4 immunoconjugate; C242 maytansinoid-4 conjugate; Cantuzumab ravtansine; IMGN-242; Maytasinoid DM4-conjugated humanised monoclonal antibody huC242; Monoclonal antibody C-242 DM4 conjugate; huC242-DM4
Click to Show/Hide
|
|||||
| Organization |
ImmunoGen, Inc.
|
|||||
| Drug Status |
Phase 2 (Terminated)
|
|||||
| Indication |
In total 3 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3 to 4
|
|||||
| Structure |
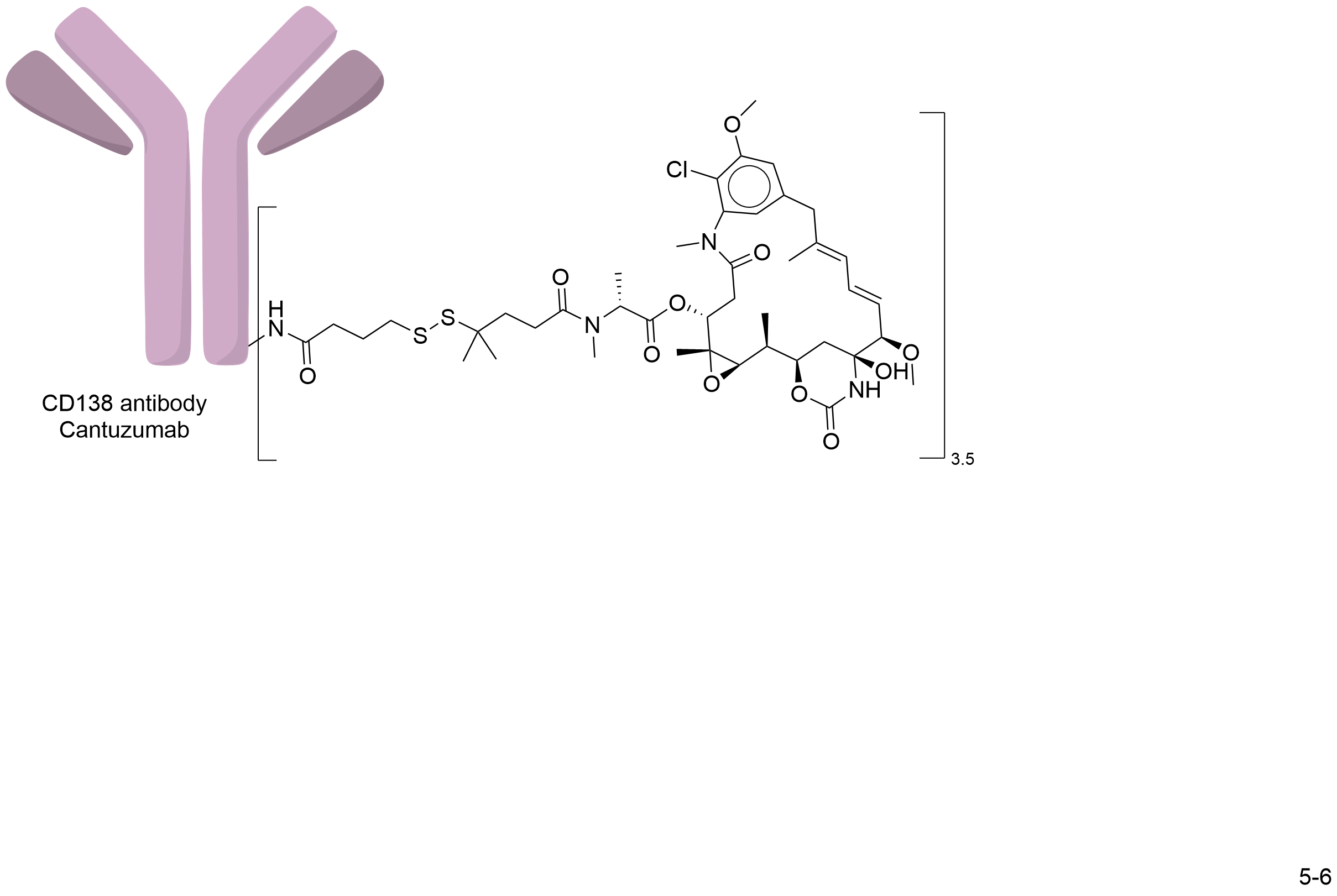
|
|||||
| Antibody Name |
Cantuzumab
|
Antibody Info | ||||
| Antigen Name |
Mucin-1 (MUC1)
|
Antigen Info | ||||
| Payload Name |
Mertansine DM4
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
N-succinimidyl 4-(2-pyridyldithio) butanoate (SPDB)
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Combination Type |
ravtansine
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Cell Line-derived Xenograft Model
| Standard Type | Value | Units | Cell Line | Disease Model |
|---|---|---|---|---|
| Tumor Growth Inhibition value (TGI) |
≈ 48.85
|
%
|
MOLP-8 cells
|
Plasma cell myeloma
|
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT00620607 | Clinical Status | Phase 2 | ||
| Clinical Description | A phase 2, open label, multiple center study of HUC242-DM4 given as an intravenous infusion once every three weeks to patients with metastatic gastric or gastroesophageal junction carcinomas. | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT00352131 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 study to assess the safety and pharmacokinetics of huC242-DM4 administered as a single intravenous infusion once every three weeks to subjects with solid tumors. | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Patients Enrolled |
Metastatic or inoperable colorectal, pancreatic, and other CanAg-expressing tumors who have failed standard therapy (about 95% of pts. had received = 4 prior chemotherapy regimens).
|
||||
| Administration Dosage |
A single intravenous (IV) infusion once every three weeks, 18, 36, 60, 90, 126, 168, 223, and 297 mg/m2.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT00352131 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 study to assess the safety and pharmacokinetics of huC242-DM4 administered as a single intravenous infusion once every three weeks to subjects with solid tumors. | ||||
| Primary Endpoint |
HuC242-DM4 was well tolerated at the 168 mg/m2 dose level. The MTD is not yet defined.
|
||||
| Other Endpoint |
No clinically significant myelosuppression and no formation of antibody to humanized antibody (HAHA) or drug (HADA).
|
||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 48.85% (Day 20) | Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
MOLP-8 cells (1.5x107 cells per mouse) suspended in a 50:50 mixture of serum free media and matrigel were injected subcutaneously in the area under the right shoulder in 100 ul. Nine groups (n=6) were treated with a single intravenous injection of ADCs, each at doses of 250 ug/kg.
|
||||
| In Vivo Model | MOLP-8 CDX model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
References
