Linker Information
General Information of This Linker
| Linker ID |
LIN0EBJON
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
Succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC)
|
|||||
| Linker Type |
Flexible dual-reactive (amino/thiol) linker
|
|||||
| Antibody-Linker Relation |
Uncleavable
|
|||||
| Structure |
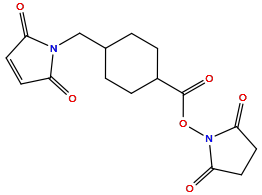
|
|||||
| Formula |
C16H18N2O6
|
|||||
| Isosmiles |
[H]C1=C([H])C(=O)N(C([H])([H])C2([H])C([H])([H])C([H])([H])C([H])(C(=O)ON3C(=O)C([H])([H])C([H])([H])C3=O)C([H])([H])C2([H])[H])C1=O
|
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C16H18N2O6/c19-12-5-6-13(20)17(12)9-10-1-3-11(4-2-10)16(23)24-18-14(21)7-8-15(18)22/h5-6,10-11H,1-4,7-9H2
|
|||||
| InChIKey |
JJAHTWIKCUJRDK-UHFFFAOYSA-N
|
|||||
| IUPAC Name |
(2,5-dioxopyrrolidin-1-yl) 4-[(2,5-dioxopyrrol-1-yl)methyl]cyclohexane-1-carboxylate
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
334.328
|
Polar area
|
101.06
|
||
|
Complexity
|
334.1164863
|
xlogp Value
|
0.325
|
|||
|
Heavy Count
|
24
|
Rot Bonds
|
4
|
|||
|
Hbond acc
|
6
|
Hbond Donor
|
0
|
|||
Each Antibody-drug Conjugate Related to This Linker
Full Information of The Activity Data of The ADC(s) Related to This Linker
Trastuzumab emtansine [Approved]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
21.40%
|
Positive HER2 expression (HER2 +++/++) | ||
| Patients Enrolled |
Untreated, asymptomatic BM or controlled brain disease treated with radiotherapy >14 days before enrollment; had received prior HER2-targeted therapy and chemotherapy; and had progressed on or after their most recent treatment of advanced breast cancer.
|
||||
| Administration Dosage |
3.6 mg/kg intravenously every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01702571 | Clinical Status | Phase 3 | ||
| Clinical Description |
A two-cohort, open-label, multicenter study of trastuzumab emtansine (T-DM1) in HER2-positive locally advanced or metastatic breast cancer patients who have received prior anti-HER2 and chemotherapy-based treatment.
|
||||
| Primary Endpoint |
Objective response rate=21.40% (95% CI 14.60-29.60), clinical benefit rate=42.90% (95% CI 34.10-52.00).
|
||||
| Other Endpoint |
Median PFS=5.50 months (95% CI, 5.30-5.60), overall survival =18.90 months (95% CI, 17.10-21.30).
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
5.10%
|
Positive HER2 expression (HER2+++/++) | ||
| Patients Enrolled |
HER2 positive advanced urothelial bladder cancer or pancreatic cancer/cholangiocarcinoma
|
||||
| Administration Dosage |
Received singleagent TDM1 2.4 mg/kg once weekly (qw) or 3.6 mg/kg every 3 weeks (q3w).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02999672 | Clinical Status | Phase 2 | ||
| Clinical Description |
A study to determine best tumor response with trastuzumab emtansine in human epidermal growth factor receptor 2 (HER2) overexpressing solid tumors (KAMELEON).
|
||||
| Primary Endpoint |
BOR, Urothelial bladder cancer (n=13); PR N=5 (38.46%);SD N=1 (7.7%);PD N=6 (46.2%);NE N=1 (7.69%). Pancreatic cancer/cholangiocarcinoma (n=7) PR N=1 (14.29%);SD N=3 (42.86%);PD N=2 (28.57%); NE N=1 (14.29%).
|
||||
| Other Endpoint |
PFS, Urothelial bladder cancer (n=13), Median PFS, months (95% CI) 2.20 (1.18-4.30), Median OS, months (95% CI) 7.03 (3.75-NE). Pancreatic cancer/cholangiocarcinoma (n=7), Median PFS, months (95% CI) 2.58 (1.31-9.99), Median OS, months (95% CI) NE (1.45-NE).
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
5.60% (Day 21)
|
|||
| Patients Enrolled |
38 patients were enrolled and 36 included in efficacy analysis.Patients were treated with the standard intravenous dosing of T- DM1, that is 3.6mg/kg every 3 weeks for a 21-day cycle.
|
||||
| Administration Dosage |
3.6 mg/kg every 3 weeks for a 21-day cycle, until toxicity or progression.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02465060 | Clinical Status | Phase 2 | ||
| Clinical Description |
Targeted therapy directed by genetic testing in treating patients with advanced refractory solid tumors, lymphomas, or multiple myeloma (the MATCH screening trial).
|
||||
| Primary Endpoint |
ORR was 2/36 (5.56%) with 90% confidence interval (90% CI, 1.00% to 16.50%)
|
||||
| Other Endpoint |
6-mouth sPFS=23.60%.
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
45.00%
|
Positive HER2 expression (HER2 +++/++) | ||
| Patients Enrolled |
An Eastern Cooperative Oncology Group performance status of 0 or 1 and centrally confirmed, measurable, HER2-positive advanced breast cancer previously treated with trastuzumab and a taxane.
|
||||
| Administration Dosage |
3.6 mg/kg ( trastuzumab emtansine) and 1200 mg (Atezolizumab) intravenously every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02924883 | Clinical Status | Phase 2 | ||
| Clinical Description |
A randomized, multicenter, double-blind, placebo-controlled phase II study of the efficacy and safety of trastuzumab emtansine in combination with atezolizumab or atezolizumab-placebo in patients with HER2-positive locally advanced or metastatic breast cancer who have received prior trastuzumab and taxane based therapy.
|
||||
| Primary Endpoint |
Median PFS=8.20 months (95% CI 5.80-10.70).
|
||||
| Other Endpoint |
Median overall survival was not estimable (95% CI NE-NE); Objective response rate=45.00% (95% CI 28.06-50.30).
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
20.00%
|
High HER2 expression (HER2+++) | ||
| Patients Enrolled |
HER2-positive, metastatic breast cancer previously treated with taxane, trastuzumab, and pertuzumab, and were T-DM1-nave.
|
||||
| Administration Dosage |
The study consisted of a dose de-escalation (dose-finding) cohort, followed by an expansion cohort at the recommended phase II dose (RP2D), T-DM1 3.60 mg/kg intravenously every 21 days, and pembrolizumab 200mg intravenously every 21 days, if one or fewer DLTs were noted in the first six patients at that dose level, it would be declared the RP2D.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03032107 | Clinical Status | Phase 1b | ||
| Clinical Description |
A phase 1b study of pembrolizumab in combination with trastuzumab-dm1 in metastatic HER2-positive breast cancer.
|
||||
| Primary Endpoint |
OrR=20.00% (95% CI 5.70%-43.70%), and median PFS=9.60 months (95%CI 2.80-16.00 months).
|
||||
| Other Endpoint |
There were no dose-limiting toxicities. The RP2D was 3.60 mg/kg T-DM1 plus 200 mg pembrolizumab every 21 days.
|
||||
| Experiment 6 Reporting the Activity Date of This ADC | [6] | ||||
| Patients Enrolled |
Newly diagnosed, HER2-positive, nonmetastatic, histologically confirmed, operable primary invasive breast carcinoma.
|
||||
| Administration Dosage |
T-DM1 was dosed at 3.60 mg/kg once every 3 weeks. Trastuzumab was dosed at 6 mg/kg once every 3 weeks after an 8 mg/kg loading dose and started concurrently with the taxane. Pertuzumab was dosed at 420 mg once every 3 weeks after an 840 mg loading dose and administered concurrently with T-DM1 or trastuzumab-plus-taxane. An interval of 3 weeks from the last dose of anthracycline to initiation of HER2-targeted therapy was required. After the taxane-concurrent phase in the trastuzumab-containing arm, trastuzumab-plus-pertuzumab was continued for 1 year. In the T-DM1containing arm, T-DM1-plus-pertuzumab was continued for 1 year.
Click to Show/Hide
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01966471 | Clinical Status | Phase 3 | ||
| Clinical Description |
A randomized, multicenter, open-label, phase 3 trial comparing trastuzumab plus pertuzumab plus a taxane following anthracyclines versus trastuzumab emtansine plus pertuzumab following anthracyclines as adjuvant therapy in patients with operable HER2-positive primary breast cancer.
|
||||
| Primary Endpoint |
82 (9.90%) IDFS events had occurred in the AC-THP arm and 80 (9.60%) had occurred in the AC-KP arm.
|
||||
| Other Endpoint |
3-year IDFS rates were 94.10% (95% CI, 92.50 to 95.70) with AC-THP and 92.80% (95% CI, 91.00 to 94.50) with AC-KP.
|
||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 1.10% (Day 28) | Low HER2 expression (HER2+) | ||
| Method Description |
The antitumor activity of T-DM1 was evaluated in various patient-derived xenograft models with different HER2 expression levels. Each cell suspension or tumor fragment was inoculated subcutaneously into specific pathogen-free female nude mice.The tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was initiated on day 0. In this group, 10 mg/kg T-DM1 was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: ST565) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 19.20% (Day 28) | Low HER2 expression (HER2+) | ||
| Method Description |
The antitumor activity of T-DM1 was evaluated in various patient-derived xenograft models with different HER2 expression levels. Each cell suspension or tumor fragment was inoculated subcutaneously into specific pathogen-free female nude mice.The tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was initiated on day 0. In this group, 10 mg/kg T-DM1 was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: ST313) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 37.60% (Day 28) | High HER2 expression (HER2+++) | ||
| Method Description |
The antitumor activity of T-DM1 was evaluated in various patient-derived xenograft models with different HER2 expression levels. Each cell suspension or tumor fragment was inoculated subcutaneously into specific pathogen-free female nude mice.The tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was initiated on day 0. In this group, 10 mg/kg T-DM1 was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX model: NIBIO G016) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 85.20% (Day 21) | Moderate HER2 expression (HER2++) | ||
| Method Description |
The antitumor activity of T-DM1 was evaluated in various patient-derived xenograft models with different HER2 expression levels. Each cell suspension or tumor fragment was inoculated subcutaneously into specific pathogen-free female nude mice.The tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was initiated on day 0. In this group, 10 mg/kg T-DM1 was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: ST225) | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.40% | Positive HER2 expression (HER2+++/++) | ||
| Method Description |
T-DM1 (Genentech) was administered i.p. at 10 mg/kg once per week.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: PDX12) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 7.10% (Day 21) | Low HER2 expression (HER2+) | ||
| Method Description |
The antitumor activity of T-DM1 was evaluated in various mice xenograft models with different HER2 expression levels; CFPAC-1 (low-expression). Each cell suspension or tumor fragment was inoculated subcutaneously into specific pathogen-free female nude mice.The tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was initiated on day 0. In this group, 10 mg/kg T-DM1 was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | CFPAC-1 cell line xenograft model | ||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | CFPAC-1 cells | CVCL_1119 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 15.50% (Day 7) | High HER2 expression (HER2+++) | ||
| Method Description |
The activity of trastuzumab-MCC-DM1 was further investigated in trastuzumab-insensitive mouse xenograft model. After transplantation of MMTV-HER2 Fo5 mammary tumor explants, tumor-bearing nude mice (n = 8 mice per group) were treated once every 3 weeks with 1 mg/kg trastuzmab-MCC-DM1.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
| Experiment 3 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 27.10% (Day 48) | High HER2 expression (HER2+++) | ||
| Method Description |
Naive female beige nude XID mice were inoculated in the mammary fat pad with 20 million tumor cells suspended in 50% phenol redfree Matrigel mixed with culture medium. All animals were randomly assigned into treatment groups, such that the mean tumor volume for each group was 100 to 200 mm3. Nude mice with established tumors were dosed i.v. with Tmab-MCC-DM1 0.3 mg/kg for a total of three injections.
Click to Show/Hide
|
||||
| In Vivo Model | Trastuzumab-resistant breast cancer CDX model | ||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-474 EEI cells | CVCL_AR96 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 31.70% (Day 21) | Low HER2 expression (HER2+) | ||
| Method Description |
The antitumor activity of T-DM1 was evaluated in various mice xenograft models with different HER2 expression levels; Capan-1 (weak positive). Each cell suspension or tumor fragment was inoculated subcutaneously into specific pathogen-free female nude mice.The tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was initiated on day 0. In this group, 10 mg/kg T-DM1 was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | Capan-1 cell line xenograft model | ||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | Capan-1 cells | CVCL_0237 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 33.10% (Day 21) | Negative HER2 expression (HER2-) | ||
| Method Description |
The antitumor activity of T-DM1 was evaluated in various mice xenograft models with different HER2 expression levels; GCIY (negative). Each cell suspension or tumor fragment was inoculated subcutaneously into specific pathogen-free female nude mice.The tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was initiated on day 0. In this group, 10 mg/kg T-DM1 was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | GCIY cell line xenograft model | ||||
| In Vitro Model | Gastric adenocarcinoma | GCIY cells | CVCL_1228 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 36.00% (Day 33) | Moderate HER2 expression (HER2++) | ||
| Method Description |
Subcutaneous injection of 100,000 cells mixed in 200 mL PBS solution was performed. Two weeks after tumour cell injection, mice were treated with trastuzumab, cetuximab or T-DM1 at a dose of 300 mg/kg via IP injection.
|
||||
| In Vivo Model | HT29 CDX model | ||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 7 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 36.00% (Day 33) | Low HER2 expression (HER2 +) | ||
| Method Description |
SW48, HT-29 and LS174T cells were injected into null mice subcutaneously, followed by treatment with cetuximab, trastuzumab and T-DM1. Two weeks after tumour cell injection, mice were treated with trastuzumab, cetuximab or T-DM1 at a dose of 300 mg/kg via IP injection.
|
||||
| In Vivo Model | SW48 CDX model | ||||
| In Vitro Model | Colon adenocarcinoma | SW48 cells | CVCL_1724 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 42.00% (Day 58) | Low HER2 expression (HER2 +) | ||
| Method Description |
11-18 cells (4,000,000 ) and HCC827 cells (2,000,000 ) were injected subcutaneously into the backs on both sides of the mice. T-DM1 (30 mg/kg, once per week i.p.) for 8 weeks.
|
||||
| In Vivo Model | 11-18 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | 11-18 cells | CVCL_6659 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 42.00% (Day 58) | Low HER2 expression (HER2+) | ||
| Method Description |
Subcutaneous injection of 100,000 cells mixed in 200 mL PBS solution was performed. Two weeks after tumour cell injection, mice were treated with trastuzumab, cetuximab or T-DM1 (30 mg/kg, once per week i.p.) for 8 weeks.
|
||||
| In Vivo Model | 11-18 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | 11-18 cells | CVCL_6659 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 43.30% (Day 30) | Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
Trastuzumab-emtansine (3 mg/kg, every seven days 4) induces efficient tumor cell killing in cell line-derived models of BT-474/R1-7 cells with HER2 expression with high expression.
|
||||
| In Vivo Model | BT-474 CDX model (T-DM1 resistant) | ||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells (Trastuzumab emtansine resistant) | CVCL_0179 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.00% (Day 21) | Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
Trastuzumab-emtansine (3 mg/kg, every seven days x3) induces efficient tumor cell killing in cell line-derived models of SK-OV-3 cells with HER2 expression with high expression.
|
||||
| In Vivo Model | SK-OV-3 CDX model (Expressing YES1 Y537F) | ||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells (YES1 Y537F expression) | CVCL_0532 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.50% (Day 48) | High HER2 expression (HER2+++) | ||
| Method Description |
Naive female beige nude XID mice were inoculated in the mammary fat pad with 20 million tumor cells suspended in 50% phenol redfree Matrigel mixed with culture medium. All animals were randomly assigned into treatment groups, such that the mean tumor volume for each group was 100 to 200 mm3. Nude mice with established tumors were dosed i.v. with Tmab-MCC-DM1 1 mg/kg for a total of three injections.
Click to Show/Hide
|
||||
| In Vivo Model | Trastuzumab-resistant breast cancer CDX model | ||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-474 EEI cells | CVCL_AR96 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 46.20% (Day 7) | High HER2 expression (HER2+++) | ||
| Method Description |
The activity of trastuzumab-MCC-DM1 was further investigated in trastuzumab-insensitive mouse xenograft model. After transplantation of MMTV-HER2 Fo5 mammary tumor explants, tumor-bearing nude mice (n = 8 mice per group) were treated once every 3 weeks with 3 mg/kg trastuzmab-MCC-DM1.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
| Experiment 14 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 55.00% (Day 33) | Positive HER2 expression (HER2+++/++) | ||
| Method Description |
Subcutaneous injection of 100,000 cells mixed in 200 mL PBS solution was performed. Two weeks after tumour cell injection, mice were treated with trastuzumab, cetuximab or T-DM1 at a dose of 300 mg/kg via IP injection.
|
||||
| In Vivo Model | HT-29 CDX model | ||||
| In Vitro Model | Colon adenocarcinoma | HT-29 cells | CVCL_0320 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 55.00% (Day 33) | Moderate HER2 expression (HER2 ++) | ||
| Method Description |
SW48, HT-29 and LS174T cells were injected into null mice subcutaneously, followed by treatment with cetuximab, trastuzumab and T-DM1. Two weeks after tumour cell injection, mice were treated with trastuzumab, cetuximab or T-DM1 at a dose of 300 mg/kg via IP injection.
|
||||
| In Vivo Model | HT-29 CDX model | ||||
| In Vitro Model | Colon adenocarcinoma | HT-29 cells | CVCL_0320 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 58.50% (Day 21) | Moderate HER2 expression (HER2++) | ||
| Method Description |
The antitumor activity of T-DM1 was evaluated in various mice xenograft models with different HER2 expression levels; JIMT-1 (moderate positive). Each cell suspension or tumor fragment was inoculated subcutaneously into specific pathogen-free female nude mice.The tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was initiated on day 0. In this group, 10 mg/kg T-DM1 was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | JIMT-1 cell line xenograft model | ||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 58.70% (Day 48) | High HER2 expression (HER2+++) | ||
| Method Description |
Naive female beige nude XID mice were inoculated in the mammary fat pad with 20 million tumor cells suspended in 50% phenol redfree Matrigel mixed with culture medium. All animals were randomly assigned into treatment groups, such that the mean tumor volume for each group was 100 to 200 mm3. Nude mice with established tumors were dosed i.v. with Tmab-MCC-DM1 3 mg/kg for a total of three injections.
Click to Show/Hide
|
||||
| In Vivo Model | Trastuzumab-resistant breast cancer CDX model | ||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-474 EEI cells | CVCL_AR96 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 63.00% (Day 58) | Positive HER2 expression (HER2+++/++) | ||
| Method Description |
Subcutaneous injection of 100,000 cells mixed in 200 mL PBS solution was performed. Two weeks after tumour cell injection, mice were treated with trastuzumab, cetuximab or T-DM1 (30 mg/kg, once per week i.p.) for 8 weeks.
|
||||
| In Vivo Model | H3255 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | NCI-H3255 cells | CVCL_6831 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 63.00% (Day 58) | High HER2 expression (HER2 +++) | ||
| Method Description |
T-DM1 (30 mg/kg, once per week i.p.) for 8 weeks,.
|
||||
| In Vivo Model | H3255 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | NCI-H3255 cells | CVCL_6831 | ||
| Experiment 20 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 63.30% (Day 33) | High HER2 expression (HER2 +++) | ||
| Method Description |
SW48, HT-29 and LS174T cells were injected into null mice subcutaneously, followed by treatment with cetuximab, trastuzumab and T-DM1. Two weeks after tumour cell injection, mice were treated with trastuzumab, cetuximab or T-DM1 at a dose of 300 mg/kg via IP injection.
|
||||
| In Vivo Model | LS174T CDX model | ||||
| In Vitro Model | Colon adenocarcinoma | LS174T cells | CVCL_1384 | ||
| Experiment 21 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 66.70% (Day 51) | Low HER2 expression (HER2+) | ||
| Method Description |
Subcutaneous injection of 100,000 cells mixed in 200 mL PBS solution was performed. Two weeks after tumour cell injection, mice were treated with trastuzumab, cetuximab or T-DM1 (30 mg/kg, once per week i.p.) for 8 weeks.
|
||||
| In Vivo Model | HCC827 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | HCC827 cells | CVCL_2063 | ||
| Experiment 22 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 74.00% (Day 7) | High HER2 expression (HER2+++) | ||
| Method Description |
The activity of trastuzumab-MCC-DM1 was further investigated in trastuzumab-insensitive mouse xenograft model. After transplantation of MMTV-HER2 Fo5 mammary tumor explants, tumor-bearing nude mice (n = 8 mice per group) were treated once every 3 weeks with 10 mg/kg trastuzmab-MCC-DM1.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
| Experiment 23 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 76.54% (Day 24) | Positive HER2 expression (HER2+++/++) | ||
| Method Description |
Inoculate 150 mice with KPL-4 cells at 3 million cells/mouse suspended in HBSS/matrigel, in the thoracic mammary fat pad at a volume of 0.2 ml. When tumors have reached a mean tumor volume of 100-250 mm3, they will be grouped out into 10 groups of 8-10 mice each. A single treatment will be administered intravenously (1 mg/kg) via the tail vein on Day 0.
Click to Show/Hide
|
||||
| In Vivo Model | KPL-4 CDX model | ||||
| In Vitro Model | Breast inflammatory carcinoma | KPL-4 cells | CVCL_5310 | ||
| Experiment 24 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 82.90% (Day 24) | Positive HER2 expression (HER2+++/++) | ||
| Method Description |
Inoculate 150 mice with KPL-4 cells at 3 million cells/mouse suspended in HBSS/matrigel, in the thoracic mammary fat pad at a volume of 0.2 ml. When tumors have reached a mean tumor volume of 100-250 mm3, they will be grouped out into 10 groups of 8-10 mice each. A single treatment will be administered intravenously (ADC-211, 1 mg/kg plus ADC-106, 5 mg/kg) via the tail vein on Day 0.
Click to Show/Hide
|
||||
| In Vivo Model | KPL-4 CDX model | ||||
| In Vitro Model | Breast inflammatory carcinoma | KPL-4 cells | CVCL_5310 | ||
| Experiment 25 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 83.90% (Day 10) | High HER2 expression (HER2+++) | ||
| Method Description |
Mice bearing mammary tumor transplants from the MMTV-HER2 Fo5 line were given a single iv injection (10 mg/kg) of Tmab-SPP-DM1, Tmab-SSNPP-DM3, Tmab-SSNPP-DM4, Tmab-MCC-DM1, or vehicle (n=7 mice per group), and tumor growth was monitored for 25 days.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
| Experiment 26 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 84.39% (Day 24) | Positive HER2 expression (HER2+++/++) | ||
| Method Description |
Inoculate 150 mice with KPL-4 cells at 3 million cells/mouse suspended in HBSS/matrigel, in the thoracic mammary fat pad at a volume of 0.2 ml. When tumors have reached a mean tumor volume of 100-250 mm3, they will be grouped out into 10 groups of 8-10 mice each. A single treatment will be administered intravenously (ADC-211, 1 mg/kg plus ADC-106, 1 mg/kg) via the tail vein on Day 0.
Click to Show/Hide
|
||||
| In Vivo Model | KPL-4 CDX model | ||||
| In Vitro Model | Breast inflammatory carcinoma | KPL-4 cells | CVCL_5310 | ||
| Experiment 27 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.70% (Day 7) | High HER2 expression (HER2+++) | ||
| Method Description |
The activity of trastuzumab-MCC-DM1 was further investigated in trastuzumab-insensitive mouse xenograft model. After transplantation of MMTV-HER2 Fo5 mammary tumor explants, tumor-bearing nude mice (n = 8 mice per group) were treated once every 3 weeks with 15 mg/kg trastuzmab-MCC-DM1.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
| Experiment 28 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.10% (Day 7) | High HER2 expression (HER2+++) | ||
| Method Description |
The activity of trastuzumab-MCC-DM1 was further investigated in trastuzumab-insensitive mouse xenograft model. After transplantation of MMTV-HER2 Fo5 mammary tumor explants, tumor-bearing nude mice (n = 8 mice per group) were treated once every 3 weeks with 30 mg/kg trastuzmab-MCC-DM1.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
| Experiment 29 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.70% (Day 48) | High HER2 expression (HER2+++) | ||
| Method Description |
Naive female beige nude XID mice were inoculated in the mammary fat pad with 20 million tumor cells suspended in 50% phenol redfree Matrigel mixed with culture medium. All animals were randomly assigned into treatment groups, such that the mean tumor volume for each group was 100 to 200 mm3. Nude mice with established tumors were dosed i.v. with Tmab-MCC-DM1 10 mg/kg for a total of three injections.
Click to Show/Hide
|
||||
| In Vivo Model | Trastuzumab-resistant breast cancer CDX model | ||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-474 EEI cells | CVCL_AR96 | ||
| Experiment 30 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 92.30% (Day 14) | High HER2 expression (HER2+++) | ||
| Method Description |
KPL-4 human breast tumor cells were inoculated (3 million cells per mouse, in Matrigel) into the mammary fat pads of SCID beige mice. All animals were randomly assigned into treatment groups, such that the mean tumor volume for each group was 100 to 200 mm3. Trastuzumab-maytansinoid conjugates were given by single 15 mg/kg iv injection.
|
||||
| In Vivo Model | Breast cancer CDX model | ||||
| In Vitro Model | Breast inflammatory carcinoma | KPL-4 cells | CVCL_5310 | ||
| Experiment 31 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.70% (Day 48) | High HER2 expression (HER2+++) | ||
| Method Description |
Naive female beige nude XID mice were inoculated in the mammary fat pad with 20 million tumor cells suspended in 50% phenol redfree Matrigel mixed with culture medium. All animals were randomly assigned into treatment groups, such that the mean tumor volume for each group was 100 to 200 mm3. Nude mice with established tumors were dosed i.v. with Tmab-MCC-DM1 15 mg/kg for a total of three injections.
Click to Show/Hide
|
||||
| In Vivo Model | Trastuzumab-resistant breast cancer CDX model | ||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-474 EEI cells | CVCL_AR96 | ||
| Experiment 32 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.40% (Day 21) | High HER2 expression (HER2+++) | ||
| Method Description |
The antitumor activity of T-DM1 was evaluated in various mice xenograft models with different HER2 expression levels; KPL-4 (strong positive). Each cell suspension or tumor fragment was inoculated subcutaneously into specific pathogen-free female nude mice.The tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was initiated on day 0. In this group, 10 mg/kg T-DM1 was i.v. to the tumor-bearing mice.
Click to Show/Hide
|
||||
| In Vivo Model | KPL-4 cell line xenograft model | ||||
| In Vitro Model | Breast inflammatory carcinoma | KPL-4 cells | CVCL_5310 | ||
| Experiment 33 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 56) | Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
HER2-positive HCC1954 cells were either treated with vehicle (control) or T-DM1 for 5 days, trypsinized, washed in PBS and sorted by flow cytometry based on the surface ROR1 expression. ROR1- and ROR1+ or unfractionated cells were injected into the subcutaneous site of 6-8-week old Nu/J mice (n=3/group).
|
||||
| In Vivo Model | HCC1954 CDX model | ||||
| In Vitro Model | Breast ductal carcinoma | HCC1954 cells | CVCL_1259 | ||
| Experiment 34 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 35) | High HER2 expression (HER2+++) | ||
| Method Description |
Mice were inoculated subcutaneously in the right flank with either 5x106 cells/mouse of BTC cell line KKU-100, mice were randomized to the control group or treatment with T-DM1 20 mg/kg groups.
|
||||
| In Vivo Model | KMCH-1 CDX model | ||||
| In Vitro Model | Combined hepatocellular carcinoma and cholangiocarcinoma | KMCH-1 cells | CVCL_7970 | ||
| Experiment 35 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Minimal Effective Dose (MED) | < 5.00 mg/kg | Moderate HER2 expression (HER2++) | ||
| Method Description |
Following the acclimatization period (1 week), the animals were stratified by body weight and randomly assigned to the following group: two T-DM1 groups, treated with 20 mg/kg and 60 mg/kg. Each group consisted of five animals for blood chemistry test as well as five animals for clinical signs and body weight measurements.
|
||||
| In Vivo Model | Gastric cancer CDX model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 36 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Maximum Tolerated Dose (MTD) | > 20.00 mg/kg | High HER2 expression (HER2+++) | ||
| Method Description |
Following the acclimatization period (1 week), the animals were stratified by body weight and randomly assigned to the following group: two T-DM1 groups, treated with 20 mg/kg and 60 mg/kg. Each group consisted of five animals for blood chemistry test as well as five animals for clinical signs and body weight measurements.
|
||||
| In Vivo Model | Gastric cancer CDX model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
Obtained from the Model Organism Data
| Experiment 1 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.98% (Day 7) | High HER2 expression (HER2 +++) | ||
| Method Description |
An allograft was propagated from the Fo5 mmty transgenic mouse which does not respond to.or responds poorly to HERCEPTIN therapy. Subjects were treated once with ADC (10 mg/kg); and placebo PBS buffer control (Vehicle) andmonitored over 3 weeks.
|
||||
| In Vivo Model | Breast cancer model MMTV-HER2 Fo5 | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [18] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [18] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.14 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.24 nM±0.12 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [18] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.26 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.57 nM±0.10 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [18] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.76 nM
|
Moderate HER2 expression (HER2 ++) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.71 nM
|
|||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. For measurement of apoptosis, BT-474 and SK-BR-3 were exposed to trastuzumab or trastuzumab-DM for 48 h.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.26 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. For measurement of apoptosis, BT-474 and SK-BR-3 were exposed to trastuzumab or trastuzumab-DM for 48 h.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.89 nM±3.51 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
6.40 nM±4.80 nM
|
Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
ADCs 21-23 was performed on HCC827 and NCI-H2228 cells. cells (5 x 103 cells/well) were cultured in 96-well plates with 100 uL complete medium, and 24 h later the cells were treated in triplicate with varying concentrations of ADCs for 72 h.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
18.37 nM
|
Low HER2 expression (HER2+; IHC 1+) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [18] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 50.00 nM | Negative HER2 expression (HER2 -) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100.00 nM | Negative HER2 expression (HER2 -) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
220.00 nM±39.50 nM
|
Negative HER2 expression (HER2 -) | ||
| Method Description |
ADCs 21-23 was performed on HCC827 and NCI-H2228 cells. cells (5 x 103 cells/well) were cultured in 96-well plates with 100 uL complete medium, and 24 h later the cells were treated in triplicate with varying concentrations of ADCs for 72 h.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
317.00 nM±93.70 nM
|
Negative HER2 expression (HER2 -) | ||
| Method Description |
ADCs 21-23 was performed on HCC827 and NCI-H2228 cells. cells (5 x 103 cells/well) were cultured in 96-well plates with 100 uL complete medium, and 24 h later the cells were treated in triplicate with varying concentrations of ADCs for 72 h.
|
||||
| In Vitro Model | Lung adenocarcinoma | HCC827 cells | CVCL_2063 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 500 nM | Negative HER2 expression (HER2 -) | ||
| Method Description |
ADCs 21-23 was performed on HCC827 and NCI-H2228 cells. cells (5 x 103 cells/well) were cultured in 96-well plates with 100 uL complete medium, and 24 h later the cells were treated in triplicate with varying concentrations of ADCs for 72 h.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
8.00-15.00 ng/mL
|
High HER2 expression (HER2+++) | ||
| Method Description |
Exposing mammalian cells having HER2 receptors to ADC in a cell culture medium; culturing the cells for a period from about 6 hours to about 5 days; and measuring cell viability Cell-based in vitro assays were used to measure viability, cytotoxicity, and induction of apoptosis of the ADC of the invention.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.00 ug/mL
|
Negative HER2 expression (HER2-) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-474 EEI cells | CVCL_AR96 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 ug/mL
|
Moderate HER2 expression (HER2++; IHC 2+) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Breast inflammatory carcinoma | KPL-4 cells | CVCL_5310 | ||
| Experiment 20 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 ug/mL
|
Positive HER2 expression (HER2+++/++; HER2 MFI=95.7) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 21 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.03 ug/mL
|
High HER2 expression (HER2+++) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Breast ductal carcinoma | HCC1954 cells | CVCL_1259 | ||
| Experiment 22 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.06 ug/mL
|
High HER2 expression (HER2+++) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | Calu-3 cells | CVCL_0609 | ||
| Experiment 23 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.27 ug/mL
|
Low HER2 expression (HER2+) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | MKN7 cells | CVCL_1417 | ||
Naratuximab emtansine [Phase 2 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
12.82%
|
Positive CD38 expression (CD38+++/++) | ||
| Patients Enrolled |
Lymphoma limited to diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), or marginal zone lymphoma (MZL). Patients were also required to have received at least one prior anti-CD20 based therapeutic regimen, have a life expectancy of greater than 3 months, an Eastern Cooperative Oncology Group Performance status of 2 or lower, and adequate hematological, renal, and hepatic function.
Click to Show/Hide
|
||||
| Administration Dosage |
Conventional 3+3 dose-escalation design; intravenously once every 3 weeks; from 0.10 to 1.80 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01534715 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1, multi-center, open-label study of IMGN529 administered intravenously in adult patients with relapsed or refractory non-Hodgkin lymphoma and chronic lymphocytic leukemia.
|
||||
| Primary Endpoint |
A total of five objective responses were observed, resulting in an overall response rate (ORR) of 12.82% (N=39). Four of these (1 complete response [CR] and 3 partial responses [PRs]) occurred in patients with DLBCL, for an ORR of 22% in this lymphoma subset.
|
||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [22] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 14.50% (Day 38) | Positive CD38 expression (CD38+++/++) | ||
| Method Description |
IMGN529 induces efficient tumor cell killing in cell line-derived models B-cell NHL of cells with lower CD27 expression, dosed at 2.5 ug/kg , 2 qw3.
|
||||
| In Vivo Model | CD37-positive NHL and CLL model | ||||
| In Vitro Model | Non-Hodgkin lymphoma | Non-Hodgkin lymphoma cells | Homo sapiens | ||
| Chronic lymphocytic leukemia | Chronic lymphocytic leukemia cells | Homo sapiens | |||
| Experiment 2 Reporting the Activity Date of This ADC | [22] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.00% (Day 38) | Positive CD38 expression (CD38+++/++) | ||
| Method Description |
IMGN529 induces efficient tumor cell killing in cell line-derived models B-cell NHL of cells with lower CD27 expression, dosed at 5 ug/kg , 2 qw3.
|
||||
| In Vivo Model | CD37-positive NHL and CLL model | ||||
| In Vitro Model | Non-Hodgkin lymphoma | Non-Hodgkin lymphoma cells | Homo sapiens | ||
| Chronic lymphocytic leukemia | Chronic lymphocytic leukemia cells | Homo sapiens | |||
| Experiment 3 Reporting the Activity Date of This ADC | [22] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 38) | Positive CD38 expression (CD38+++/++) | ||
| Method Description |
IMGN529 induces efficient tumor cell killing in cell line-derived models B-cell NHL of cells with lower CD27 expression, dosed at 10 ug/kg , 2 qw3.
|
||||
| In Vivo Model | CD37-positive NHL and CLL model | ||||
| In Vitro Model | Non-Hodgkin lymphoma | Non-Hodgkin lymphoma cells | Homo sapiens | ||
| Chronic lymphocytic leukemia | Chronic lymphocytic leukemia cells | Homo sapiens | |||
| Experiment 4 Reporting the Activity Date of This ADC | [22] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (10 mg/kg)
|
Positive CD38 expression (CD38+++/++) | ||
| Method Description |
The inhibitory activity of SGN-LIV1A against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 1 day.
|
||||
| In Vivo Model | DoHH2 CDX model | ||||
| In Vitro Model | Diffuse large B-cell lymphoma | WSU-NHL cells | CVCL_1793 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [22] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.10 nM | |||
| Method Description |
In vitro cytotoxicity was measured by incubating 5000 target cells with indicated agents in complete RPMI-1640 media for 5 days at 37°C. The viability of remaining cells was determined by a colorimetric WST-10 assay.
|
||||
| In Vivo Model | DoHH2 CDX model | ||||
| In Vitro Model | Diffuse large B-cell lymphoma germinal center B-cell type | DoHH2 cells | CVCL_1179 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [22] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.10 nM | |||
| Method Description |
In vitro cytotoxicity was measured by incubating 5000 target cells with indicated agents in complete RPMI-1640 media for 5 days at 37°C. The viability of remaining cells was determined by a colorimetric WST-11 assay.
|
||||
| In Vivo Model | DoHH2 CDX model | ||||
| In Vitro Model | Mantle cell lymphoma | Granta-519 cells | CVCL_1818 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [22] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.10 nM | |||
| Method Description |
In vitro cytotoxicity was measured by incubating 5000 target cells with indicated agents in complete RPMI-1640 media for 5 days at 37°C. The viability of remaining cells was determined by a colorimetric WST-8 assay.
|
||||
| In Vivo Model | DoHH2 CDX model | ||||
| In Vitro Model | Burkitt lymphoma | BJAB cells | CVCL_5711 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [22] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.10 nM | Positive CD38 expression (CD38+++/++) | ||
| Method Description |
In vitro cytotoxicity was measured by incubating 5000 target cells with indicated agents in complete RPMI-1640 media for 5 days at 37°C. The viability of remaining cells was determined by a colorimetric WST-9 assay.
|
||||
| In Vivo Model | DoHH2 CDX model | ||||
| In Vitro Model | Diffuse large B-cell lymphoma | Farage cells | CVCL_3302 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [22] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 1.00 nM | |||
| Method Description |
In vitro cytotoxicity was measured by incubating 5000 target cells with indicated agents in complete RPMI-1640 media for 5 days at 37°C. The viability of remaining cells was determined by a colorimetric WST-12 assay.
|
||||
| In Vivo Model | DoHH2 CDX model | ||||
| In Vitro Model | Mantle cell lymphoma | JVM-2 cells | CVCL_1319 | ||
AVID-100 [Phase 1/2 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [23] | ||||
| Patients Enrolled |
Patients with advanced or metastatic epithelial malignancies.
|
||||
| Administration Dosage |
20, 40, 80, 120, 180, and 220 mg/m2 every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03094169 | Clinical Status | Phase 1/2 | ||
| Clinical Description |
Phase 1a/2a dose escalation trial to determine safety, tolerance, MTD, and preliminary antineoplastic activity of AVID100, in patients with advanced or metastatic solid tumors of epithelial origin.
|
||||
B-003 [Phase 1]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [24] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03953833 | Clinical Status | Phase 1 | ||
| Clinical Description |
Phase 1 clinical trial on the safety, tolerability, pharmacokinetics of B003 in the treatment of HER2-positive recurrent or metastatic breast cancer.
|
||||
PCA-062 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [25] | ||||
| Efficacy Data | Disease Control Rate (DCR) |
22.60% (31 patients with other tumors)
33.30% (HNSCC) 22.20% (esophageal cancer) |
|||
| Patients Enrolled |
Advanced solid tumors expressing P-cadherin, TNBC, head and neck squamous cell carcinoma (HNSCC), esophageal cancer, cervical cancer, and non-small cell lung cancer (NSCLC).
|
||||
| Administration Dosage |
At 10 different dose levels of PCA062, ranging from 0.40 to 5.00 mg/kg every 2 weeks administered as a 1-hour intravenous infusion.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02375958 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1 multi-center, open-label dose escalation and expansion study of PCA062 administered intravenously in adult patients with p-CAD positive tumors.
|
||||
| Primary Endpoint |
The MTD was PCA062 3.60 mg/kg every 2 weeks.No patient achieved a complete response. Only 1 patient with stage IV metastatic HNSCC treated at 0.90 mg/kg achieved a confirmed partial response (PR) as best overall response (BOR). The disease control rate (DCR) for the 31 patients with other tumors was 22.60% (95% CI, 9.60-41.10). In patients with HNSCC (n = 6), DCR was 33.30% (95% CI, 4.30-77.70), and in patients with esophageal cancer (n = 9), DCR was 22.20% (95% CI, 2.80-60.00).
Click to Show/Hide
|
||||
Laprituximab emtansine [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [26] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT01963715 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1, multi-center, open-label study of IMGN289 administered intravenously in adult patients with EGFR-positive solid tumors.
|
||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [29] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
58.30%
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
The cytotoxic activity of IMGN289 was evaluated against a panel of SCCHN cell lines in vitro. Immunodeficient mice bearing established subcutaneous xenograft tumors were treated with a single intravenous injection of IMGN289 at 1,2.5 or 5.0 mg/kg (based on antibody concentration). IMGN289 dose was 5 mg/kg administered as a single injection.
|
||||
| In Vivo Model | Head and neck squamous cell carcinomas CDX model | ||||
| In Vitro Model | Hypopharyngeal squamous cell carcinoma | FaDu cells | CVCL_1218 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [29] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
83.30%
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
The cytotoxic activity of IMGN289 was evaluated against a panel of SCCHN cell lines in vitro. Immunodeficient mice bearing established subcutaneous xenograft tumors were treated with a single intravenous injection of IMGN289 at 1,2.5 or 5.0 mg/kg (based on antibody concentration). IMGN289 dose was 5 mg/kg administered as a single injection.
|
||||
| In Vivo Model | Head and neck squamous cell carcinomas CDX model | ||||
| In Vitro Model | Oral cavity squamous cell carcinoma | HSC-2 cells | CVCL_1287 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [29] | ||||
| Efficacy Data | Minimal Effective Dose (MED) |
1.00 mg/kg
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
The cytotoxic activity of IMGN289 was evaluated against a panel of SCCHN cell lines in vitro. Immunodeficient mice bearing established subcutaneous xenograft tumors were treated with a single intravenous injection of IMGN289 at 1,2.5 or 5.0 mg/kg (based on antibody concentration).
|
||||
| In Vivo Model | Head and neck squamous cell carcinomas CDX model | ||||
| In Vitro Model | Hypopharyngeal squamous cell carcinoma | FaDu cells | CVCL_1218 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [29] | ||||
| Efficacy Data | Minimal Effective Dose (MED) |
2.50 mg/kg
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
The cytotoxic activity of IMGN289 was evaluated against a panel of SCCHN cell lines in vitro. Immunodeficient mice bearing established subcutaneous xenograft tumors were treated with a single intravenous injection of IMGN289 at 1,2.5 or 5.0 mg/kg (based on antibody concentration).
|
||||
| In Vivo Model | Head and neck squamous cell carcinomas CDX model | ||||
| In Vitro Model | Oral cavity squamous cell carcinoma | HSC-2 cells | CVCL_1287 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [30] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.25 nM
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
Effects of IMGN289 on clonogenicity and proliferation were tested in HNSCC cell lines with varying cetuximab and gefitinib sensitivities.
|
||||
| In Vitro Model | Head and neck squamous cell carcinoma | HNSCC cells | Homo sapiens | ||
SHR-A1201 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [27] | ||||
| Patients Enrolled |
HER2-positive locally advanced or metastatic breast cancer (positivity for HER2 was defined as a score of 2+ in immunohistochemical analysis and a positive result in fluorescence in situ hybridization or a score of 3+ in immunohistochemical analysis), the standard treatment was invalid or there was no effective standard treatment plan, the Eastern Cooperative Oncology Group performance status score was 0-1.
Click to Show/Hide
|
||||
| Administration Dosage |
4 dose-escalation sequences, 1.20 mg/kg, 2.40 mg/kg, 3.60 mg/kg and 4.80 mg/kg, once every 21days, as a 90-min intravenous infusion.
|
||||
LOP-628 [Phase 1 (Terminated)]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [28] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 43.30% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive GIST430 xenograft model. Animals were administered a single i.v. injection of the vehicle, IgG-ADC, or LOP628, LMJ729, imatinib.
|
||||
| In Vivo Model | KIT-expressing GIST430 xenograft model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [28] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 46.00% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive NCI-H1048 SCLC xenograft model. Animals were administered a single i.v. injection of the vehicle, IgG-ADC, or LOP628; twice daily 80 mg/kg oral doses of imatinib or the combination of LOP628 with imatinib.
|
||||
| In Vivo Model | KIT-expressing NCI-H1048 SCLC xenograft model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [28] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 75.00% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive GIST430 xenograft model. Animals were administered a single i.v. injection of the vehicle, IgG-ADC, or LOP628, LMJ729, imatinib.
|
||||
| In Vivo Model | KIT-expressing GIST430 xenograft model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [28] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.20% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive GIST-T1 xenograft model. Animals were administered a single i.v. injection of the vehicle, IgG-ADC, or LOP628; twice daily 80 mg/kg oral doses of imatinib or the combination of LOP628 with imatinib.
|
||||
| In Vivo Model | KIT-expressing GIST-T1 xenograft model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [28] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
82.20%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive GIST-T1 xenograft model. An efficacy study (single 0.625 mg/kg dose) in the GIST-T1 xenograft model in mice was performed.
|
||||
| In Vivo Model | KIT-expressing GIST-T1 xenograft model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [28] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.30% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive NCI-H1048 SCLC xenograft model. Animals were administered a single i.v. injection of the vehicle, IgG-ADC, or LOP628; twice daily 80 mg/kg oral doses of imatinib or the combination of LOP628 with imatinib.
|
||||
| In Vivo Model | KIT-expressing NCI-H1048 SCLC xenograft model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [28] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.30% | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The in vivo antitumor activity of LOP-628 was evaluated in a c-KIT positive NCI-H1048 SCLC xenograft model. Animals were administered a single i.v. injection of the vehicle, IgG-ADC, or LOP628; twice daily 80 mg/kg oral doses of imatinib or the combination of LOP628 with imatinib.
|
||||
| In Vivo Model | KIT-expressing NCI-H1048 SCLC xenograft model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [28] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) | < 0.50 nM | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The inhibitory activity of LOP-628 against cancer cell growth was compared with LMJ729 against cancer cell growth in vitro.
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [28] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) | < 1.00 nM | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The inhibitory activity of LOP-628 against cancer cell growth was compared with LMJ729 against cancer cell growth in vitro.
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [28] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) | > 10.00 nM | Negative KIT expression (KIT-) | ||
| Method Description |
The inhibitory activity of LOP-628 against cancer cell growth was compared with LMJ729 against cancer cell growth in vitro.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
Anti-FGFR2/4 mAb-12425 SMCC-DM1 [Investigative]
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 9.18% (Day 44) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 208.4 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 5 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | Breast cancer PDX model | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 60.63% (Day 30) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 208.4 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 10 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: CHGA-010) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 73.73% (Day 30) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 208.4 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 10 mg/kg intravenous (i.v, q3w x2) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: CHGA-010) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 81.30% (Day 44) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 208.4 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 15 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | Breast cancer PDX model | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 52.51% (Day 38) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 208.4 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 10 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | MFM223 CDX model | ||||
| In Vitro Model | Breast carcinoma | MFM-223 cells | CVCL_1408 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 63.93% (Day 22) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 171.7 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 5 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H716 CDX model | ||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H716 cells | CVCL_1581 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 66.08% (Day 22) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 171.7 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 15 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H716 CDX model | ||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H716 cells | CVCL_1581 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.41% (Day 33) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.1-0.7 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H716 cells | CVCL_1581 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.15 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.17 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Breast carcinoma | SUM52PE cells | CVCL_3425 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.20 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Breast carcinoma | MFM-223 cells | CVCL_1408 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Negative FGFR2 expression (FGFR2-) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric carcinoma | NUGC-3 cells | CVCL_1612 | ||
C12G1-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [32] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 30) | High NECTIN2 expression (NECTIN2+++) | ||
| Method Description |
Treatments c12G1-DM1 at 1 mg/kg every 4th day by intraperitoneal injection.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [32] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 30) | High NECTIN2 expression (NECTIN2+++) | ||
| Method Description |
Treatments c12G1-DM1 at 5 mg/kg every 4th day by intraperitoneal injection.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [32] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 10.40% (Day 30) | High NECTIN2 expression (NECTIN2+++) | ||
| Method Description |
Treatments c12G1-DM1 at 3 mg/kg every 4th day by intraperitoneal injection.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [32] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 11.50% (Day 30) | High NECTIN2 expression (NECTIN2+++) | ||
| Method Description |
Treatments c12G1-DM1 at 1 mg/kg every 4th day by intraperitoneal injection.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian cancer | Ovarian cancer cells | Homo sapiens | ||
| Experiment 5 Reporting the Activity Date of This ADC | [32] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 19.60% (Day 30) | High NECTIN2 expression (NECTIN2+++) | ||
| Method Description |
Treatments c12G1-DM1 at 5 mg/kg every 4th day by intraperitoneal injection.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian cancer | Ovarian cancer cells | Homo sapiens | ||
| Experiment 6 Reporting the Activity Date of This ADC | [32] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 32.90% (Day 30) | High NECTIN2 expression (NECTIN2+++) | ||
| Method Description |
Treatments c12G1-DM1 at 1 mg/kg every 4th day by intraperitoneal injection.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian adenocarcinoma | OV-90 cells | CVCL_3768 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [32] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 46.40% (Day 30) | High NECTIN2 expression (NECTIN2+++) | ||
| Method Description |
Treatments c12G1-DM1 at 3 mg/kg every 4th day by intraperitoneal injection.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian cancer | Ovarian cancer cells | Homo sapiens | ||
| Experiment 8 Reporting the Activity Date of This ADC | [32] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 84.40% (Day 30) | High NECTIN2 expression (NECTIN2+++) | ||
| Method Description |
Treatments c12G1-DM1 at 3 mg/kg every 4th day by intraperitoneal injection.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian adenocarcinoma | OV-90 cells | CVCL_3768 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [32] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.90% (Day 30) | High NECTIN2 expression (NECTIN2+++) | ||
| Method Description |
Treatments c12G1-DM1 at 5 mg/kg every 4th day by intraperitoneal injection.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian adenocarcinoma | OV-90 cells | CVCL_3768 | ||
NN2101-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 2.01% (Day 20) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The animal room had a controlled 12/12-h light/dark cycle (lights on at 06:00 AM), temperature (20±26 °C), and relativehumidity (50±10%). For xenograft assays, mice were anesthetized with isoflurane. Then, cells in 50% Matrigel were subcutaneously transplanted into 5-week-old female C.B-17 severe combined immunodeficiency (SCID) mice. In vivo efficacy studies were initiated when the volume of the tumors reached ~ 200 mm3. Imatinib was formulated in distilled water for oral administration. The mice were intravenously administered NN2101-DM1 three times at the indicated concentrations,2 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | GIST xenograft model | ||||
| In Vitro Model | Mast cell leukemia | HMC-1.2 cells | CVCL_H205 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 12.30% (Day 50) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The animal room had a controlled 12/12-h light/dark cycle (lights on at 06:00 AM), temperature (20±26 °C), and relativehumidity (50±10%). For xenograft assays, mice were anesthetized with isoflurane. Then, cells in 50% Matrigel were subcutaneously transplanted into 5-week-old female C.B-17 severe combined immunodeficiency (SCID) mice. In vivo efficacy studies were initiated when the volume of the tumors reached ~ 200 mm3. Imatinib was formulated in distilled water for oral administration. The mice were intravenously administered NN2101-DM1 three times at the indicated concentrations,1 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | GIST xenograft model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 20.50% (Day 27) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The animal room had a controlled 12/12-h light/dark cycle (lights on at 06:00 AM), temperature (20±26 °C), and relativehumidity (50±10%). For xenograft assays, mice were anesthetized with isoflurane. Then, cells in 50% Matrigel were subcutaneously transplanted into 5-week-old female C.B-17 severe combined immunodeficiency (SCID) mice. In vivo efficacy studies were initiated when the volume of the tumors reached ~ 200 mm3. Imatinib was formulated in distilled water for oral administration. The mice were intravenously administered NN2101-DM1 three times at the indicated concentrations,3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | SCLC xenograft model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 50.40% (Day 50) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The animal room had a controlled 12/12-h light/dark cycle (lights on at 06:00 AM), temperature (20±26 °C), and relativehumidity (50±10%). For xenograft assays, mice were anesthetized with isoflurane. Then, cells in 50% Matrigel were subcutaneously transplanted into 5-week-old female C.B-17 severe combined immunodeficiency (SCID) mice. In vivo efficacy studies were initiated when the volume of the tumors reached ~ 200 mm3. Imatinib was formulated in distilled water for oral administration. The mice were intravenously administered NN2101-DM1 three times at the indicated concentrations,1 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | GIST xenograft model | ||||
| In Vitro Model | Adult hepatocellular carcinoma | Huh-7 cells | CVCL_0336 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 61.30% (Day 60) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The animal room had a controlled 12/12-h light/dark cycle (lights on at 06:00 AM), temperature (20±26 °C), and relativehumidity (50±10%). For xenograft assays, mice were anesthetized with isoflurane. Then, cells in 50% Matrigel were subcutaneously transplanted into 5-week-old female C.B-17 severe combined immunodeficiency (SCID) mice. In vivo efficacy studies were initiated when the volume of the tumors reached ~ 200 mm3. Imatinib was formulated in distilled water for oral administration. The mice were intravenously administered NN2101-DM1 three times at the indicated concentrations,3.5 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | GIST xenograft model | ||||
| In Vitro Model | Mast cell leukemia | HMC-1.2 cells | CVCL_H205 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 61.30% (Day 60) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The animal room had a controlled 12/12-h light/dark cycle (lights on at 06:00 AM), temperature (20±26 °C), and relativehumidity (50±10%). For xenograft assays, mice were anesthetized with isoflurane. Then, cells in 50% Matrigel were subcutaneously transplanted into 5-week-old female C.B-17 severe combined immunodeficiency (SCID) mice. In vivo efficacy studies were initiated when the volume of the tumors reached ~ 200 mm3. Imatinib was formulated in distilled water for oral administration. The mice were intravenously administered NN2101-DM1 three times at the indicated concentrations,5 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | GIST xenograft model | ||||
| In Vitro Model | Mast cell leukemia | HMC-1.2 cells | CVCL_H205 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.90% (Day 50) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The animal room had a controlled 12/12-h light/dark cycle (lights on at 06:00 AM), temperature (20±26 °C), and relativehumidity (50±10%). For xenograft assays, mice were anesthetized with isoflurane. Then, cells in 50% Matrigel were subcutaneously transplanted into 5-week-old female C.B-17 severe combined immunodeficiency (SCID) mice. In vivo efficacy studies were initiated when the volume of the tumors reached ~ 200 mm3. Imatinib was formulated in distilled water for oral administration. The mice were intravenously administered NN2101-DM1 three times at the indicated concentrations,3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | GIST xenograft model | ||||
| In Vitro Model | Adult hepatocellular carcinoma | Huh-7 cells | CVCL_0336 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.10% (Day 50) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The animal room had a controlled 12/12-h light/dark cycle (lights on at 06:00 AM), temperature (20±26 °C), and relativehumidity (50±10%). For xenograft assays, mice were anesthetized with isoflurane. Then, cells in 50% Matrigel were subcutaneously transplanted into 5-week-old female C.B-17 severe combined immunodeficiency (SCID) mice. In vivo efficacy studies were initiated when the volume of the tumors reached ~ 200 mm3. Imatinib was formulated in distilled water for oral administration. The mice were intravenously administered NN2101-DM1 three times at the indicated concentrations,3 mg/kg. The mice were intraperitoneally administered Etoposide was prepared in dimethyl sulfoxide, and the solution was diluted in PBS just before administration. The mice were intraperitoneally administered etoposide for 2 cycles (3 mg/kg per day;days 15 and days 1115).
Click to Show/Hide
|
||||
| In Vivo Model | GIST xenograft model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.30% (Day 27) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
The animal room had a controlled 12/12-h light/dark cycle (lights on at 06:00 AM), temperature (20±26 °C), and relativehumidity (50±10%). For xenograft assays, mice were anesthetized with isoflurane. Then, cells in 50% Matrigel were subcutaneously transplanted into 5-week-old female C.B-17 severe combined immunodeficiency (SCID) mice. In vivo efficacy studies were initiated when the volume of the tumors reached ~ 200 mm3. Imatinib was formulated in distilled water for oral administration. The mice were intravenously administered NN2101-DM1 three times at the indicated concentrations,3 mg/kg. The mice were intraperitoneally administered Etoposide was prepared in dimethyl sulfoxide, and the solution was diluted in PBS just before administration. The mice were intraperitoneally administered etoposide for 2 cycles (3 mg/kg per day;days 15 and days 1115).
Click to Show/Hide
|
||||
| In Vivo Model | SCLC xenograft model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.38 ng/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.43 ng/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-430/654 cells | CVCL_7040 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.02 ng/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
8.07 ng/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.77 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Mast cell leukemia | HMC-1.2 cells | CVCL_H205 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.18 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.52 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | Caov-3 cells | CVCL_0201 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.54 ug/mL
|
Negative KIT expression (KIT-) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.99 ug/mL
|
Negative KIT expression (KIT-) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.71 ug/mL
|
Negative KIT expression (KIT-) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Lung squamous cell carcinoma | NCI-H2170 cells | CVCL_1535 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.03 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.30 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.47 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
7.38 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Esophageal squamous cell carcinoma | TF-1 cells | CVCL_1759 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
11.84 ug/mL
|
Negative KIT expression (KIT-) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Normal | COS-7 cells | CVCL_0224 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
11.95 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Normal | HUVEC-C cells | CVCL_2959 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
12.47 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H889 cells | CVCL_1598 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
17.49 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST48 cells | CVCL_7041 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [33] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
17.63 ug/mL
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
For apoptosis assays, the cells were seeded into 96-well plates and incubated in a humidified CO2 chamber for 16 h. The cells were incubated with vehicle, NN2101-DM1 (1g/mL) for 72 h.
|
||||
| In Vitro Model | Lung small cell carcinoma | Ms-1 cells | CVCL_IQ55 | ||
Anti-FGFR2 mAb-10164 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 2.05% (Day 22) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 171.7 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 5 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H716 CDX model | ||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H716 cells | CVCL_1581 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 57.22% (Day 22) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 171.7 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 15 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H716 CDX model | ||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H716 cells | CVCL_1581 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 65.68% (Day 38) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 208.4 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 10 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | MFM223 CDX model | ||||
| In Vitro Model | Breast carcinoma | MFM-223 cells | CVCL_1408 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.61% (Day 33) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.14 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Breast carcinoma | SUM52PE cells | CVCL_3425 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.50 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.60 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.70 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H716 cells | CVCL_1581 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.50 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Breast carcinoma | MFM-223 cells | CVCL_1408 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Negative FGFR2 expression (FGFR2-) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric carcinoma | NUGC-3 cells | CVCL_1612 | ||
T-DM1-IR700 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 10.10% (Day 7) | Negative HER2 expression (HER2 -) | ||
| Method Description |
Tra-IR700 (3.6 ug/g) or T-DM1-IR700 (3.6 ug/g) was administered intravenously to mice on Day 1 (without NIR light, 6 days after tumor cell transplantation). The dose similar to that of T-DM1 administered to humans (3.6 mg/kg). The NIR-light was irradiated at 1 and 2 days after the drug administration.
|
||||
| In Vivo Model | MDA-MB-468GFP CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468GFP cells | CVCL_DH83 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 36.40% (Day 7) | Negative HER2 expression (HER2 -) | ||
| Method Description |
Tra-IR700 (3.6 ug/g) or T-DM1-IR700 (3.6 ug/g) was administered intravenously to mice on Day 1 (without NIR light, 6 days after tumor cell transplantation). The dose similar to that of T-DM1 administered to humans (3.6 mg/kg). The NIR-light was irradiated at 1 and 2 days after the drug administration.
|
||||
| In Vivo Model | MDA-MB-468GFP CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468GFP cells | CVCL_DH83 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM - 10 nM
|
Positive HER2 expression (HER2+++/++) | ||
| Method Description |
Cells (100,000) were seeded in 12-well dishes and incubated with Tra-IR700 (10 ug/ml) or T-DM1-IR700 (10 ug/ml) for 6 h at 37°C. For NIR-PIT, cells were irradiated with 4 J/cm2 of NIR light from a 690 nm-Laser.
|
||||
| In Vitro Model | Lung squamous cell carcinoma | NCI-H2170 cells | CVCL_1535 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM - 10 nM
|
Positive HER2 expression (HER2+++/++) | ||
| Method Description |
Cells (100,000) were seeded in 12-well dishes and incubated with Tra-IR700 (10 ug/ml) or T-DM1-IR700 (10 ug/ml) for 6 h at 37°C. For NIR-PIT, cells were irradiated with 4 J/cm2 of NIR light from a 690 nm-Laser.
|
||||
| In Vitro Model | Lung adenocarcinoma | Calu-3 cells | CVCL_0609 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM - 10 nM
|
Low HER2 expression (HER2+) | ||
| Method Description |
Cells (100,000) were seeded in 12-well dishes and incubated with Tra-IR700 (10 ug/ml) or T-DM1-IR700 (10 ug/ml) for 6 h at 37°C. For NIR-PIT, cells were irradiated with 4 J/cm2 of NIR light from a 690 nm-Laser.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM - 10 nM
|
Positive HER2 expression (HER2+++/++) | ||
| Method Description |
Cells (100,000) were seeded in 12-well dishes and incubated with Tra-IR700 (10 ug/ml) or T-DM1-IR700 (10 ug/ml) for 6 h at 37°C. For NIR-PIT, cells were irradiated with 4 J/cm2 of NIR light from a 690 nm-Laser.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.50 nM
|
Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
Cells (100,000) were seeded in 12-well dishes and incubated with Tra-IR700 (10 ug/ml) or T-DM1-IR700 (10 ug/ml) for 6 h at 37°C. For NIR-PIT, cells were irradiated with 4 J/cm2 of NIR light from a 690 nm-Laser.
|
||||
| In Vitro Model | Breast adenocarcinoma | 3T3/HER2-luc-GFP cells | Mus musculus | ||
| Experiment 6 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
100 nM
|
Negative HER2 expression (HER2 -) | ||
| Method Description |
Cells (100,000) were seeded in 12-well dishes and incubated with Tra-IR700 (10 ug/ml) or T-DM1-IR700 (10 ug/ml) for 6 h at 37°C. For NIR-PIT, cells were irradiated with 4 J/cm2 of NIR light from a 690 nm-Laser.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468GFP cells | CVCL_DH83 | ||
F105-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [34] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 17.58% (Day 49) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
The rear flank region of female athymic rats were implanted with 5x106 cells subcutaneously (0.2 ml of25 x106 cells/ml) on the rear flank area. When mean tumor volumes reached to 250 mm3, dosed intravenously (15 mg/kg) on days 17 and 29 after tumor cell injection.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
Anti-KIT NEG085?SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 20.12% (Day 28) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (1.25 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST430 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 32.28% (Day 38) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (1.25 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 39.65% (Day 28) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST430 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 69.09% (Day 28) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST430 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 72.00% (Day 41) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (0.625 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 75.90% (Day 38) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.25% (Day 38) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 79.31% (Day 21) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (1.25 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H526 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 81.81% (Day 27) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.0 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.44% (Day 21) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H526 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.51% (Day 21) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H526 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
59.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
71.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
76.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
80.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
90.00%
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
98.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.03 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.47 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.55 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.64 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.82 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
46.83 nM
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
HcHAb18-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [36] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 23.23% (Day 27) | Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
HcHAb18-DM1 (1 mg/kg, twice a week for 4 weeks) induces efficient tumor cell killing in cell line-derived models of A549 cells with CD147 expression with high expression.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [36] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 23.23% (Day 27) | Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
HcHAb18-DM1 (2 mg/kg, twice a week for 4 weeks) induces efficient tumor cell killing in cell line-derived models of A549 cells with CD147 expression with high expression.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [36] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 38.63% (Day 27) | Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
HcHAb18-DM1 (4 mg/kg, twice a week for 4 weeks) induces efficient tumor cell killing in cell line-derived models of A549 cells with CD147 expression with high expression.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [36] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 56.95% (Day 27) | Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
HcHAb18-DM1 (8 mg/kg, twice a week for 4 weeks) induces efficient tumor cell killing in cell line-derived models of A549 cells with CD147 expression with high expression.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [36] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 89.22% (Day 27) | Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
HcHAb18-DM1 (16 mg/kg, twice a week for 4 weeks) induces efficient tumor cell killing in cell line-derived models of A549 cells with CD147 expression with high expression.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [36] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.2% (Day 29) | Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
HcHAb18-DM1 (30 mg/kg, i.v. injection weekly in a total 4 doses) induces efficient tumor cell killing in cell line-derived models of NCI-H226 cells with CD147 expression with high expression.
|
||||
| In Vivo Model | NCI-H226 CDX model | ||||
| In Vitro Model | Pleural epithelioid mesothelioma | NCI-H226 cells | CVCL_1544 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [36] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.84% (Day 27) | Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
HcHAb18-DM1 (32 mg/kg, twice a week for 4 weeks) induces efficient tumor cell killing in cell line-derived models of A549 cells with CD147 expression with high expression.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [36] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.40% (Day 14) | Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
HcHAb18-DM1 (18 mg/kg, twice a week for 4 weeks) induces efficient tumor cell killing in cell line-derived models of NCI-H460 cells with CD147 expression with high expression.
|
||||
| In Vivo Model | NCI-H460 CDX model | ||||
| In Vitro Model | Lung large cell carcinoma | NCI-H460 cells | CVCL_0459 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [36] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.46 ug/mL
|
Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
In vitro efficacy of HcHAb18-DM1 versus HcHAb18 and IgG1-DM1 on six cell lines treated for 72 h.
|
||||
| In Vitro Model | Pleural epithelioid mesothelioma | NCI-H226 cells | CVCL_1544 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [36] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.77 ug/mL
|
Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
In vitro efficacy of HcHAb18-DM1 versus HcHAb18 and IgG1-DM1 on six cell lines treated for 72 h.
|
||||
| In Vitro Model | Lung large cell carcinoma | NCI-H460 cells | CVCL_0459 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [36] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.96 ug/mL
|
Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
In vitro efficacy of HcHAb18-DM1 versus HcHAb18 and IgG1-DM1 on six cell lines treated for 72 h.
|
||||
| In Vitro Model | Lung squamous cell carcinoma | NCI-H520 cells | CVCL_1566 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [36] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
18.26 ug/mL
|
Positive CD147 expression (CD147 +++/++) | ||
| Method Description |
In vitro efficacy of HcHAb18-DM1 versus HcHAb18 and IgG1-DM1 on six cell lines treated for 72 h.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
CNTO95-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [34] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 28.74% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 3 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [34] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 44.54% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 6 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [34] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.23% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 10 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [34] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.81% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 15 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
Anti-FGFR2/4 mAb-12422 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 30.85% (Day 22) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 171.7 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 5 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H716 CDX model | ||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H716 cells | CVCL_1581 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 35.79% (Day 22) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 171.7 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 15 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H716 CDX model | ||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H716 cells | CVCL_1581 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.52% (Day 33) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
4C9-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [37] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 40.00% (Day 14) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
In vivo efficacy of 4C9-DM1 was examined using mouse models xenotransplanted with NCI-H526, mice with established tumors were randomized into different treatment groups when the tumor volume reached ~200 mm 3. The dose of 4C9-DM1 was 1 mg/kg twice at day 0 and day 7.
|
||||
| In Vivo Model | Small cell lung cancer CDX model | ||||
| In Vitro Model | Small cell lung cancer | Small cell lung cancer cells | Homo sapiens | ||
| Experiment 2 Reporting the Activity Date of This ADC | [37] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.00% (Day 14) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
In vivo efficacy of 4C9-DM1 was examined using mouse models xenotransplanted with NCI-H526, mice with established tumors were randomized into different treatment groups when the tumor volume reached ~200 mm 3. The dose of 4C9-DM1 was 3 mg/kg twice at day 0 and day 7.
|
||||
| In Vivo Model | Small cell lung cancer CDX model | ||||
| In Vitro Model | Small cell lung cancer | Small cell lung cancer cells | Homo sapiens | ||
| Experiment 3 Reporting the Activity Date of This ADC | [37] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 59.00% (Day 14) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
In vivo efficacy of 4C9-DM1 was examined using mouse models xenotransplanted with NCI-H526, mice with established tumors were randomized into different treatment groups when the tumor volume reached ~200 mm 3. The dose of 4C9-DM1 was 5 mg/kg twice at day 0 and day 7.
|
||||
| In Vivo Model | Small cell lung cancer CDX model | ||||
| In Vitro Model | Small cell lung cancer | Small cell lung cancer cells | Homo sapiens | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [37] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.16 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
C-Kit positive or negative SCLC cell lines were seeded into 96-well plates and incubated with 4C9 or 4C9-DM1 in a dose-dependent manner for 35 days.
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [37] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.32 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
C-Kit positive or negative SCLC cell lines were seeded into 96-well plates and incubated with 4C9 or 4C9-DM1 in a dose-dependent manner for 35 days.
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H889 cells | CVCL_1598 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [37] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.08 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
C-Kit positive or negative SCLC cell lines were seeded into 96-well plates and incubated with 4C9 or 4C9-DM1 in a dose-dependent manner for 35 days.
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [37] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
16.58 nM
|
Negative KIT expression (KIT-) | ||
| Method Description |
C-Kit positive or negative SCLC cell lines were seeded into 96-well plates and incubated with 4C9 or 4C9-DM1 in a dose-dependent manner for 35 days.
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H446 cells | CVCL_1562 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [37] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
35.50 nM
|
Negative KIT expression (KIT-) | ||
| Method Description |
C-Kit positive or negative SCLC cell lines were seeded into 96-well plates and incubated with 4C9 or 4C9-DM1 in a dose-dependent manner for 35 days.
|
||||
| In Vitro Model | Lung squamous cell carcinoma | NCI-H2170 cells | CVCL_1535 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [37] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
47.63 nM
|
Negative KIT expression (KIT-) | ||
| Method Description |
C-Kit positive or negative SCLC cell lines were seeded into 96-well plates and incubated with 4C9 or 4C9-DM1 in a dose-dependent manner for 35 days.
|
||||
| In Vitro Model | Amelanotic melanoma | MDA-MB-435 cells | CVCL_0417 | ||
Anti-KIT 20376?SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 43.00% (Day 23) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (10 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 49.00% (Day 40) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (0.625 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 91.00% (Day 40) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.00% (Day 40) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.00% (Day 40) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (10 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
54.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
69.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
69.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
79.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
95.00%
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
97.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
100.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.05 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.33 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.34 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.51 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.69 nM
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
Anti-KIT NEG087?SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 44.00% (Day 41) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (0.625 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
57.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
70.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
78.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
80.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
90.00%
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
98.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.03 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.05 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.41 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.50 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.24 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
6.07 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
45.43 nM
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
Anti-FGFR2 mAb-12433 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 44.97% (Day 38) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 208.4 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 10 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | MFM223 CDX model | ||||
| In Vitro Model | Breast carcinoma | MFM-223 cells | CVCL_1408 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 91.03% (Day 33) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.69% (Day 31) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 35) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 233 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 15 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.29 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Breast carcinoma | SUM52PE cells | CVCL_3425 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
24.00 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Breast carcinoma | MFM-223 cells | CVCL_1408 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Cecum adenocarcinoma | NCI-H716 cells | CVCL_1581 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Negative FGFR2 expression (FGFR2-) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric carcinoma | NUGC-3 cells | CVCL_1612 | ||
Anti-KIT NEG026?SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.00% (Day 41) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (0.625 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
TF-mAb-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [38] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 48.00% (Day 20) | Positive TF expression (TF +++) | ||
| Method Description |
Conjugates of the exemplary antibodies were tested using an established xenograft model implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 3.75 mg/kg, i.v., qw of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | HCC1806 CDX model | ||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [38] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 20) | Positive TF expression (TF +++) | ||
| Method Description |
Conjugates of the exemplary antibodies were tested using an established xenograft model implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 15 mg/kg, i.v., qw of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | HCC1806 CDX model | ||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [38] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.02 nM
|
Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [38] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | BxPC-3 cells | CVCL_0186 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [38] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07 nM
|
Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Invasive breast carcinoma | Hs 578T cells | CVCL_0332 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [38] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [38] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
17.20 nM
|
Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Glioblastoma | U-87MG cells | CVCL_0022 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [38] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
25.40 nM
|
Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [38] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [38] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [38] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
100 nM
|
Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [38] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Positive TF expression (TF +++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Invasive breast carcinoma | T-47D cells | CVCL_0553 | ||
Mirvetuximab-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [39] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
55.86% (Day 14)
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Animals with established tumors of about 130 mm3 were treated with intravenous single injection of the M9346A-DM conjugates at 25 02 mg/kg, equivalent to 51 3 g conjugated maytansinoid per kg The conjugates were injected on day 7 after cell inoculation.
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [39] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
99.17% (Day 10)
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Five-week-old female CB-17 severe combined immunodeficient (SCID) mice were obtained and quarantined for 7 days prior to study initiation Mice were inoculated subcutaneously with KB cells (1 x107 cells per mouse) resuspended in serum-free culture mediaMice with established KB xenografts were dosed with a single intravenous injection of 200 g of conjugated maytansinoid per kg, equivalent to 102 mg/kg antibody on day 6 after cell inoculationTumor dimensions were measured twice weekly and volume was calculated as (A B2) 05, where A represents the largest and B the perpendicular tumor diameter.
Click to Show/Hide
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [39] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.08±0.01 nM
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [39] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10±0.02 nM
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [39] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
7.00±2.00 nM
|
Moderate FOLR1 expression (FOLR1++; 290,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure) Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Gestational choriocarcinoma | JEG-3 cells | CVCL_0363 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [39] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
20.00±0.70 nM
|
High FOLR1 expression (FOLR1+++; 1,300,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
huMov19-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [40] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 56.67% (Day 20) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 2.5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [40] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 89.15% (Day 20) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [40] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.52% (Day 20) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 10 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [40] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [40] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [40] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [40] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
20.00 nM
|
Moderate FOLR1 expression (FOLR1 ++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Gestational choriocarcinoma | JEG-3 cells | CVCL_0363 | ||
Anti-KIT NEG086?SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 57.00% (Day 41) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (0.625 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.61% (Day 27) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.0 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
54.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
69.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
74.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
80.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
91.00%
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
98.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.03 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.06 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.43 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.52 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.83 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.22 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
16.92 nM
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
Anti-FGFR2/4 mAb-10918 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 61.12% (Day 33) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.20 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.61 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
Anti-FGFR2/4 mAb-11722 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 61.76% (Day 33) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.20 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.59 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
Anti-KIT NEG027?SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 63.65% (Day 42) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (0.625 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 83.95% (Day 42) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (1.25 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 85.25% (Day 42) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
HuFR1-65-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [39] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
65.00% (Day 12)
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Five-week-old female CB-17 severe combined immunodeficient (SCID) mice were obtained and quarantined for 7 days prior to study initiation Mice were inoculated subcutaneously with KB cells (1 x107 cells per mouse) resuspended in serum-free culture mediaMice with established KB xenografts were dosed with a single intravenous injection of 200 g of conjugated maytansinoid per kg, equivalent to 102 mg/kg antibody on day 6 after cell inoculationTumor dimensions were measured twice weekly and volume was calculated as (A B2) 05, where A represents the largest and B the perpendicular tumor diameter.
Click to Show/Hide
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [39] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.08±0.02 nM
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
HuFR1-48-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [39] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
65.00% (Day 12)
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Five-week-old female CB-17 severe combined immunodeficient (SCID) mice were obtained and quarantined for 7 days prior to study initiation Mice were inoculated subcutaneously with KB cells (1 x107 cells per mouse) resuspended in serum-free culture mediaMice with established KB xenografts were dosed with a single intravenous injection of 200 g of conjugated maytansinoid per kg, equivalent to 102 mg/kg antibody on day 6 after cell inoculationTumor dimensions were measured twice weekly and volume was calculated as (A B2) 05, where A represents the largest and B the perpendicular tumor diameter.
Click to Show/Hide
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [39] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07±0.02 nM
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Anti-FGFR2 mAb-12944 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 65.73% (Day 31) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
Anti-FGFR2 mAb-10846 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 71.12% (Day 31) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.20 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.43 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
Anti-FGFR2/4 mAb-10923 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 71.79% (Day 33) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.20 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.45 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
Anti-FGFR2 mAb-11723 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 74.86% (Day 31) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.20 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.91 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
Anti-FGFR2 mAb-10220 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 76.72% (Day 33) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.20 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.33 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
Anti-KIT NEG024?SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.00% (Day 41) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (0.625 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.01% (Day 27) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.0 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.00% (Day 23) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 23) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 23) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (10 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
53.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
66.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
72.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
78.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
92.00%
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
98.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.02 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.72 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST882 cells | CVCL_7044 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.74 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.26 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
8.49 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
22.77 nM
|
Negative KIT expression (KIT-) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
Anti-FGFR2 mAb-11725 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.87% (Day 31) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.00 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.20 nM
|
Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
nBT062-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [41] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 78.02% (Day 30) | Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
MOLP-8 cells (1.5x107 cells per mouse) suspended in a 50:50 mixture of serum free media and matrigel were injected subcutaneously in the area under the right shoulder in 100 ul. Nine groups (n=6) were treated with a single intravenous injection of ADCs, each at doses of 250 ug/kg.
|
||||
| In Vivo Model | MOLP-8 CDX model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [41] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.32% (Day 30) | Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
MOLP-8 cells (1.5x107 cells per mouse) suspended in a 50:50 mixture of serum free media and matrigel were injected subcutaneously in the area under the right shoulder in 100 ul. Nine groups (n=6) were treated with a single intravenous injection of ADCs, each at doses of 250 ug/kg per week (five weeks).
|
||||
| In Vivo Model | MOLP-8 CDX model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [41] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.27% (Day 30) | Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
MOLP-8 cells (1.5x107 cells per mouse) suspended in a 50:50 mixture of serum free media and matrigel were injected subcutaneously in the area under the right shoulder in 100 ul. Nine groups (n=6) were treated with a single intravenous injection of ADCs, each at doses of 450 ug/kg.
|
||||
| In Vivo Model | MOLP-8 CDX model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [41] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM-1.00 nM
|
Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
CD138+ MOLP-8 cells were seeded in flat bottom plates at 3000 cells/well. CD138- BJAB control cells were seeded at 1000 cells/weli. The cells were treated with nBT062-SPDB-DM4nBT062-SPP-DM1 or nBT062-SMCC-DM1 at different concentrations for five days.
|
||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [41] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Negative CD138 expression (CD138 -) | ||
| Method Description |
CD138+ MOLP-8 cells were seeded in flat bottom plates at 3000 cells/well. CD138- BJAB control cells were seeded at 1000 cells/weli. The cells were treated with nBT062-SPDB-DM4nBT062-SPP-DM1 or nBT062-SMCC-DM1 at different concentrations for five days.
|
||||
| In Vitro Model | Burkitt lymphoma | BJAB cells | CVCL_5711 | ||
Anti-KIT 9P3?SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 78.93% (Day 14) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with Kasumi-1 cells. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (10 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | Kasumi-1 CDX model | ||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 84.35% (Day 35) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with Kasumi-1 cells. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (10 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | HMC-1 CDX model | ||||
| In Vitro Model | Mast cell leukemia | HMC-1 cells | CVCL_0003 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.00% (Day 43) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (10 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST430 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 50) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 50) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (10 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | GIST-T1 CDX model | ||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
60.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
75.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
86.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H889 cells | CVCL_1598 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
87.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1930 cells | CVCL_1507 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
91.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute megakaryoblastic leukemia | CMK-11-5 cells | CVCL_0217 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
92.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
95.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Erythroleukemia | HEL 92.1.7 cells | CVCL_2481 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
98.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | OCI-M1 cells | CVCL_2149 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Adult acute myeloid leukemia | SKNO-1 cells | CVCL_2196 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Essential thrombocythemia | UKE-1 cells | CVCL_0104 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
100.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
100.00%
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute megakaryoblastic leukemia | M-07e cells | CVCL_2106 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.02 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST-T1 cells | CVCL_4976 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.05 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute megakaryoblastic leukemia | CMK-11-5 cells | CVCL_0217 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.05 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H526 cells | CVCL_1569 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.08 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute megakaryoblastic leukemia | M-07e cells | CVCL_2106 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.08 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Gastrointestinal stromal tumor | GIST430 cells | CVCL_7040 | ||
| Experiment 20 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.09 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1930 cells | CVCL_1507 | ||
| Experiment 21 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.11 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | OCI-M1 cells | CVCL_2149 | ||
| Experiment 22 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.15 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H889 cells | CVCL_1598 | ||
| Experiment 23 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
0.61 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Erythroleukemia | HEL 92.1.7 cells | CVCL_2481 | ||
| Experiment 24 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
1.29 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Acute myeloid leukemia | Kasumi-6 cells | CVCL_0614 | ||
| Experiment 25 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
1.80 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Essential thrombocythemia | UKE-1 cells | CVCL_0104 | ||
| Experiment 26 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
3.60 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Adult acute myeloid leukemia | SKNO-1 cells | CVCL_2196 | ||
| Experiment 27 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
4.00 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Myeloid leukemia with maturation | Kasumi-1 cells | CVCL_0589 | ||
| Experiment 28 Reporting the Activity Date of This ADC | [35] | ||||
| Efficacy Data | Half Maximum Growth Inhibitory Concentration (GI50) |
4.30 nM
|
Positive KIT expression (KIT+++/++) | ||
| Method Description |
Cells were cultured in a tissue culture incubator at 37°C with 5% CO2 in culture medium. On the day of the assay, cells were washed twice with PBS, prior to being treated with 0.1% trypsin-EDTA for 5 min and resuspended in the recommended culture medium. Cells were then counted and seeded in 96 well plates at densities of 2,000-10,000 cells/well in 100 ul of cell culture medium.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
Anti-FGFR2/4 mAb-12439 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.60% (Day 31) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
HuFR1-49-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [39] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
85.00% (Day 12)
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Five-week-old female CB-17 severe combined immunodeficient (SCID) mice were obtained and quarantined for 7 days prior to study initiation Mice were inoculated subcutaneously with KB cells (1 x107 cells per mouse) resuspended in serum-free culture mediaMice with established KB xenografts were dosed with a single intravenous injection of 200 g of conjugated maytansinoid per kg, equivalent to 102 mg/kg antibody on day 6 after cell inoculationTumor dimensions were measured twice weekly and volume was calculated as (A B2) 05, where A represents the largest and B the perpendicular tumor diameter.
Click to Show/Hide
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [39] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07±0.01 nM
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Anti-FGFR2 mAb-12931 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 85.56% (Day 31) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
HuFR1-57-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [39] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
86.67% (Day 12)
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Five-week-old female CB-17 severe combined immunodeficient (SCID) mice were obtained and quarantined for 7 days prior to study initiation Mice were inoculated subcutaneously with KB cells (1 x107 cells per mouse) resuspended in serum-free culture mediaMice with established KB xenografts were dosed with a single intravenous injection of 200 g of conjugated maytansinoid per kg, equivalent to 102 mg/kg antibody on day 6 after cell inoculationTumor dimensions were measured twice weekly and volume was calculated as (A B2) 05, where A represents the largest and B the perpendicular tumor diameter.
Click to Show/Hide
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [39] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.15±0.02 nM
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
Anti-FGFR2 mAb-12947 SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.66% (Day 31) | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
The total injection volume containing cells in suspension was 200 ul. Mice were enrolled in the first study eight days post implantation with average tumor volume of 192.9 mm3. After being randomly assigned to one of nine groups (n =8/group), mice were administered PBS or a single 3 mg/kg intravenous (i.v,) injection of one antibody drug conjugates.
Click to Show/Hide
|
||||
| In Vivo Model | SNU-16 CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Gastric adenocarcinoma | SNU-16 cells | CVCL_0076 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [31] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 3.00 nM | Positive FGFR2 expression (FGFR2+++/++) | ||
| Method Description |
Cells were counted and seeded in 96 well plates at densities of 2600-3600 cells/well in 100 ul of cell culture medium, A duplicate plate was generated for a day 0 measurement and all plates were incubated in a tissue culture incubator at 37°C with 5% CO2 overnight. Following this incubation, 50 ul/well of Cell titer Glo reagent was added to the day 0 plates, which were then shaken gently for 10 min and the resulting luminescence intensity was measured.
Click to Show/Hide
|
||||
| In Vitro Model | Down syndrome | KATO III cells | CVCL_0371 | ||
M9346A-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [40] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 20) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OVCAR-3 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 10 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
FR1-48-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [40] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.05 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
FR1-65-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [40] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
FR1-57-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [40] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
FR1-49-SMCC-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [40] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
T-DM1-2.6 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [42] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
Positive HER2 expression (HER2+++/++) | ||
| Method Description |
Cells were cultured in a 10% fetal bovine serum (FBS)-containing RPMI 1640 medium and were planted into 96-well plates with 6000 cells per well. The plates were incubated overnight at 37°C in a 5% CO2 cell incubator.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [42] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.05 nM
|
Positive HER2 expression (HER2+++/++) | ||
| Method Description |
Cells were cultured in a 10% fetal bovine serum (FBS)-containing RPMI 1640 medium and were planted into 96-well plates with 6000 cells per well. The plates were incubated overnight at 37°C in a 5% CO2 cell incubator.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [42] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 66.00 nM | Negative HER2 expression (HER2 -) | ||
| Method Description |
Cells were cultured in a 10% fetal bovine serum (FBS)-containing RPMI 1640 medium and were planted into 96-well plates with 6000 cells per well. The plates were incubated overnight at 37°C in a 5% CO2 cell incubator.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
H32-DM1_3.8 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.05 nM±0.05 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.54 nM±0.07 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.76 nM±0.15 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
H32-DM1_3.3 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.05 nM±0.02 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.65 nM±0.04 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.65 nM±0.05 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
H32-DM1_3.7 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.06 nM±0.04 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.75 nM±0.06 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.79 nM±0.24 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
H32-DM1_3.0 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.08 nM±0.01 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.77 nM±0.15 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [19] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.84 nM±0.15 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
To determine the IC50 values of the ADCs, different concentrations of each conjugate was directly added to the culture medium. After the cells were cultured for 3 days at 37°C, the number of viable cells was quantified by using the WST- reagent, and the absorbance was measured at OD450.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
5E3-emtansine [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [43] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.21 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | MM1.S cells | CVCL_8792 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [43] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.42 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | NCI-H929 cells | CVCL_1600 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [43] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.30 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | INA-6 cells | CVCL_5209 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [43] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.80 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | OCI-My7 cells | CVCL_E333 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [43] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
9.90 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | OCI-My5 cells | CVCL_E332 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [43] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
12.90 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | KMS-12-BM cells | CVCL_1334 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [43] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
16.90 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Multiple myeloma | SK-MM-1 cells | CVCL_A478 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [43] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
35.30 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | OPM-2 cells | CVCL_1625 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [43] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
37.00 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | JIM3 cells | CVCL_2533 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [43] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
39.60 nM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | LP-1 cells | CVCL_0012 | ||
Trastuzumab-MCC-CpG conjugate [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.27 nM
|
Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
Human HER2 positive or negative cell lines and B14.3 HER2 cell lines were seeded at 1x104 cells per well in 96-well plates. Serial dilutions of vehicle control, isotype control, ODN, Trastuzumab and Trastuzumab-ODN conjugates were added and cells were incubated at 37°C, 5% CO2 for 48h.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.43 nM
|
Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
Human HER2 positive or negative cell lines and B14.3 HER2 cell lines were seeded at 1x104 cells per well in 96-well plates. Serial dilutions of vehicle control, isotype control, ODN, Trastuzumab and Trastuzumab-ODN conjugates were added and cells were incubated at 37°C, 5% CO2 for 48h.
|
||||
| In Vitro Model | Esophageal adenocarcinoma | OE19 cells | CVCL_1622 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [44] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Negative HER2 expression (HER2 -) | ||
| Method Description |
Human HER2 positive or negative cell lines and B14.3 HER2 cell lines were seeded at 1x104 cells per well in 96-well plates. Serial dilutions of vehicle control, isotype control, ODN, Trastuzumab and Trastuzumab-ODN conjugates were added and cells were incubated at 37°C, 5% CO2 for 48h.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
EGFRvIII-MCC-DM1 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [45] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.40 nM
|
Positive EGFR vIII expression (EGFR vIII+++/++) | ||
| Method Description |
Cells were seeded in PDL-coated 96 well plates at 375 for MMT/hEGFRvIII, 1500 for U251/hEGFRvIII, 2000 for HEK293/hEGFRvIII, or 3000 for C4-2, PC3, and T47D cells per well in complete growth media and grown overnight.
|
||||
| In Vitro Model | Normal | HEK293 cells | CVCL_0045 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [45] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.00 nM
|
Positive EGFR vIII expression (EGFR vIII+++/++) | ||
| Method Description |
Cells were seeded in PDL-coated 96 well plates at 375 for MMT/hEGFRvIII, 1500 for U251/hEGFRvIII, 2000 for HEK293/hEGFRvIII, or 3000 for C4-2, PC3, and T47D cells per well in complete growth media and grown overnight.
|
||||
| In Vitro Model | Astrocytoma | U-251MG cells | CVCL_0021 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [45] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
10.00 nM
|
Positive EGFR vIII expression (EGFR vIII+++/++) | ||
| Method Description |
Cells were seeded in PDL-coated 96 well plates at 375 for MMT/hEGFRvIII, 1500 for U251/hEGFRvIII, 2000 for HEK293/hEGFRvIII, or 3000 for C4-2, PC3, and T47D cells per well in complete growth media and grown overnight.
|
||||
| In Vitro Model | Malignant neoplasms of the mouse mammary gland | MMT-060562 cells | CVCL_4241 | ||
Cet-DM1 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [46] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.85 nM
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
HCC1954 cells (20,000) were plated in 150 mm dishes and treated with 1 nM T-DM1 (media replaced weekly). Resistant HCC1954 T-DM1R clones (HCC-TDM1R#) were obtained by single-cell cloning and continuously cultured in the presence of 1 nM T-DM1 for a total period of 5 months.
|
||||
| In Vivo Model | HCC1954/TDR CDX model (CDX: C#10) | ||||
| In Vitro Model | Breast ductal carcinoma | HCC1954 T-DM1R (T-DM1 resistance) cells | CVCL_1259 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [46] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.85 nM
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
HCC1954 cells (20,000) were plated in 150 mm dishes and treated with 1 nM T-DM1 (media replaced weekly). Resistant HCC1954 T-DM1R clones (HCC-TDM1R#) were obtained by single-cell cloning and continuously cultured in the presence of 1 nM T-DM1 for a total period of 5 months.
|
||||
| In Vivo Model | HCC1954/TDR CDX model (CDX: C#19) | ||||
| In Vitro Model | Breast ductal carcinoma | HCC1954 T-DM1R (T-DM1 resistance) cells | CVCL_1259 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [46] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.41 nM
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
HCC1954 cells (20,000) were plated in 150 mm dishes and treated with 1 nM T-DM1 (media replaced weekly). Resistant HCC1954 T-DM1R clones (HCC-TDM1R#) were obtained by single-cell cloning and continuously cultured in the presence of 1 nM T-DM1 for a total period of 5 months.
|
||||
| In Vitro Model | Breast ductal carcinoma | HCC1954 cells | CVCL_1259 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [46] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.52 nM
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
HCC1954 cells (20,000) were plated in 150 mm dishes and treated with 1 nM T-DM1 (media replaced weekly). Resistant HCC1954 T-DM1R clones (HCC-TDM1R#) were obtained by single-cell cloning and continuously cultured in the presence of 1 nM T-DM1 for a total period of 5 months.
|
||||
| In Vivo Model | HCC1954/TDR CDX model (CDX: C#20) | ||||
| In Vitro Model | Breast ductal carcinoma | HCC1954 T-DM1R (T-DM1 resistance) cells | CVCL_1259 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [46] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
7.68 nM
|
High EGFR expression (EGFR+++/++) | ||
| Method Description |
HCC1954 cells (20,000) were plated in 150 mm dishes and treated with 1 nM T-DM1 (media replaced weekly). Resistant HCC1954 T-DM1R clones (HCC-TDM1R#) were obtained by single-cell cloning and continuously cultured in the presence of 1 nM T-DM1 for a total period of 5 months.
|
||||
| In Vivo Model | HCC1954/TDR CDX model (CDX: C#29) | ||||
| In Vitro Model | Breast ductal carcinoma | HCC1954 T-DM1R (T-DM1 resistance) cells | CVCL_1259 | ||
B4-SMCC-DC4 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.20 nM
|
Positive CD19 expression (CD19+++/++) | ||
| Method Description |
Cytotoxicity of B4-SPP-DCx Conjugates (with acid phosphatase treatment) against Ramos (Ag+) and HL60/s (Ag-) Cells.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [47] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.60 nM
|
Negative CD19 expression(CD19-) | ||
| Method Description |
Cytotoxicity of B4-SPP-DCx Conjugates (with acid phosphatase treatment) against Ramos (Ag+) and HL60/s (Ag-) Cells.
|
||||
| In Vitro Model | Adult acute myeloid leukemia | HL-60 cells | CVCL_0002 | ||
ICAM1-DM1 ADC [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.00 nM
|
Positive ICAM1 expression (ICAM1+++/++) | ||
| Method Description |
Cells were plated in 96-well tissue culture plates (SigmaAldrich) 1 day before treatment, serum-starved, and treated with serially diluted Tras-Me-PRX (10 mg/mL antibody) for 24 h.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | BxPC-3 cells | CVCL_0186 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
9.80 nM
|
Positive ICAM1 expression (ICAM1+++/++) | ||
| Method Description |
Cells were plated in 96-well tissue culture plates (SigmaAldrich) 1 day before treatment, serum-starved, and treated with serially diluted Tras-Me-PRX (10 mg/mL antibody) for 24 h.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | PANC-1 cells | CVCL_0480 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [48] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Positive ICAM1 expression (ICAM1+++/++) | ||
| Method Description |
Cells were plated in 96-well tissue culture plates (SigmaAldrich) 1 day before treatment, serum-starved, and treated with serially diluted Tras-Me-PRX (10 mg/mL antibody) for 24 h.
|
||||
| In Vitro Model | Normal | hTERT-HPNE cells | CVCL_C466 | ||
40H3-SMCC-DM1 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [49] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
6.65 nM
|
High EGFR expression (EGFR+++) | ||
| Method Description |
1 x104 cells per well in a volume of 100 ul were plated in 96-well tissue culture plates. After 24 h, ADCs were added at the indicated concentrations. After 72 h, the medium was removed and the viability was determined using the CellTiter-Glo luminescent cell viability assay kit.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [49] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
89.60 nM
|
High EGFR expression (EGFR+++) | ||
| Method Description |
1 x104 cells per well in a volume of 100 ul were plated in 96-well tissue culture plates. After 24 h, ADCs were added at the indicated concentrations. After 72 h, the medium was removed and the viability was determined using the CellTiter-Glo luminescent cell viability assay kit.
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [49] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100.00 nM | Moderate EGFR expression (EGFR++) | ||
| Method Description |
1 x104 cells per well in a volume of 100 ul were plated in 96-well tissue culture plates. After 24 h, ADCs were added at the indicated concentrations. After 72 h, the medium was removed and the viability was determined using the CellTiter-Glo luminescent cell viability assay kit.
|
||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-20 cells | CVCL_0178 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [49] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100.00 nM | Negative EGFR expression (EGFR-) | ||
| Method Description |
1 x104 cells per well in a volume of 100 ul were plated in 96-well tissue culture plates. After 24 h, ADCs were added at the indicated concentrations. After 72 h, the medium was removed and the viability was determined using the CellTiter-Glo luminescent cell viability assay kit.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
GABRP Ab-DM1 ADC [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
100 ng/mL - 500 ng/mL
|
Positive GABRP expression (GABRP+++/++) | ||
| Method Description |
Cells were plated at 2000 cells/well in triplicate in 96-well plates and exposed to GABRP Ab-DM1 ADC and control IgG-DM1 for 3 days.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
100 ng/mL - 500 ng/mL
|
Positive GABRP expression (GABRP+++/++) | ||
| Method Description |
Cells were plated at 2000 cells/well in triplicate in 96-well plates and exposed to GABRP Ab-DM1 ADC and control IgG-DM1 for 3 days.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 500 ng/mL | Positive GABRP expression (GABRP+++/++) | ||
| Method Description |
Cells were plated at 2000 cells/well in triplicate in 96-well plates and exposed to GABRP Ab-DM1 ADC and control IgG-DM1 for 3 days.
|
||||
| In Vitro Model | Breast ductal carcinoma | HCC1143 cells | CVCL_1245 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 500 ng/mL | Positive GABRP expression (GABRP+++/++) | ||
| Method Description |
Cells were plated at 2000 cells/well in triplicate in 96-well plates and exposed to GABRP Ab-DM1 ADC and control IgG-DM1 for 3 days.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [50] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 500 ng/mL | Positive GABRP expression (GABRP+++/++) | ||
| Method Description |
Cells were plated at 2000 cells/well in triplicate in 96-well plates and exposed to GABRP Ab-DM1 ADC and control IgG-DM1 for 3 days.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
Y-TR1 SMCC [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [51] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
15.00 ug/mL
|
Positive CD26 expression (CD26+++/++) | ||
| Method Description |
Viability assay of Jurkat cells incubated for 72 h with ADC.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
References
