Linker Information
General Information of This Linker
| Linker ID |
LIN0QDUUQ
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
N-succinimidyl 4-(2-pyridyldithio) pentanoate (SPP)
|
|||||
| Linker Type |
Thiol-sensitive linker
|
|||||
| Antibody-Linker Relation |
Cleavable
|
|||||
| Structure |
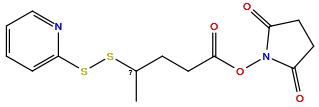
|
|||||
| Formula |
C14H16N2O4S2
|
|||||
| Isosmiles |
[H]c1nc(SSC([H])(C([H])([H])[H])C([H])([H])C([H])([H])C(=O)ON2C(=O)C([H])([H])C([H])([H])C2=O)c([H])c([H])c1[H]
|
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C14H16N2O4S2/c1-10(21-22-11-4-2-3-9-15-11)5-8-14(19)20-16-12(17)6-7-13(16)18/h2-4,9-10H,5-8H2,1H3
|
|||||
| InChIKey |
GTBCXYYVWHFQRS-UHFFFAOYSA-N
|
|||||
| IUPAC Name |
(2,5-dioxopyrrolidin-1-yl) 4-(pyridin-2-yldisulfanyl)pentanoate
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
340.426
|
Polar area
|
76.57
|
||
|
Complexity
|
340.055149
|
xlogp Value
|
2.5978
|
|||
|
Heavy Count
|
22
|
Rot Bonds
|
8
|
|||
|
Hbond acc
|
7
|
Hbond Donor
|
0
|
|||
Each Antibody-drug Conjugate Related to This Linker
Full Information of The Activity Data of The ADC(s) Related to This Linker
Lorvotuzumab mertansine [Phase 2 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
28.30%
|
Positive CD56 expression (CD56+++/++) | ||
| Patients Enrolled |
Relapsed and/or Refractory CD-56-positive Multiple Myeloma.
|
||||
| Administration Dosage |
40 mg/m2 (up to a maximum of 140 mg) intravenously once every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT00346255 | Clinical Status | Phase 1 | ||
| Clinical Description |
BB-10901 in treating patients with relapsed and/or refractory multiple myeloma (IMGN901).
|
||||
| Primary Endpoint |
Objective response rate=28.30%.
|
||||
| Other Endpoint |
Median progression-free survival=26.10 months (95% CI 1-89 weeks).
|
||||
Bivatuzumab mertansine [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
0.00%
|
|||
| Patients Enrolled |
Metastatic breast cancer (MBC) that expresses CD44v6 in at least 50% of tumor cells in primary tumor tissue as assessed by immunohistochemistry, pretreatment with anthracyclines and taxanes, tumor metastases measurable by computed tomography (CT) or magnetic resonance imaging (MRI), life expectancy of at least 6 months, no chemotherapy, radiotherapy or immunotherapy within the last 4 weeks before study entry, adequate organ function, Eastern Cooperative Oncology Group performance score 2.
Click to Show/Hide
|
||||
| Administration Dosage |
One single intravenous infusion over 30 min, dose was escalated in 25 mg/m2 increments up to the maximum tolerated dose (MTD).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02254005 | Clinical Status | Phase 1 | ||
| Clinical Description |
An open phase 1 single dose escalation study of bivatuzumab mertansine administered intravenously in female patients with CD44v6 positive metastatic breast cancer with repeated administration in patients with clinical benefit.
|
||||
| Primary Endpoint |
The MTD in this trial could not be determined.
|
||||
| Other Endpoint |
No objective responses were observed. Disease stabilization was achieved in 50.00% of patients independently of dose level.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02254044 | Clinical Status | Phase 1 | ||
| Clinical Description |
An open phase 1 dose escalation study of bivatuzumab mertansine administered intravenously once per week for three weeks in patients with advanced squamous cell carcinoma of the head and neck or esophagus with repeated administration courses in patients with clinical benefit.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [5] | ||||
| Patients Enrolled |
Ecurrent or metastatic head and neck squamous cell carcinoma (HNSCC) not amenable to established treatments, an ECOG score 2, and an estimated life expectancy of at least 6 months, a tumor diameter of at least 1 cm in CT or MRI scans was also required.
|
||||
| Administration Dosage |
Starting with 25 mg/m2, the dose was escalated in steps of 25 mg/m2 until dose limiting toxicity was observed, intravenously.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02254044 | Clinical Status | Phase 1 | ||
| Clinical Description |
An open phase 1 dose escalation study of bivatuzumab mertansine administered intravenously once per week for three weeks in patients with advanced squamous cell carcinoma of the head and neck or esophagus with repeated administration courses in patients with clinical benefit.
|
||||
| Primary Endpoint |
The MTD was 300 mg/m2.
|
||||
| Other Endpoint |
Due to the premature discontinuation of the trial efficacy complying with the study plan could not be assessed. In 3 patients,a partial response at doses of 2.00, 2.75 and 3.25 mg/m2.
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [6] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02254031 | Clinical Status | Phase 1 | ||
| Clinical Description |
An open phase 1 dose escalation study of bivatuzumab mertansine administered intravenously once per week for three weeks in female patients with CD44V6 positive recurrent or metastatic breast cancer with repeated administration courses in patients with clinical.
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [7] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02254018 | Clinical Status | Phase 1 | ||
| Clinical Description |
An open phase 1 single dose escalation study of bivatuzumab mertansine administered intravenously in patients with advanced squamous cell carcinoma of the head and neck with repeated administration in patients with clinical benefit.
|
||||
| Experiment 6 Reporting the Activity Date of This ADC | [8] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02254005 | Clinical Status | Phase 1 | ||
| Clinical Description |
An open phase 1 single dose escalation study of bivatuzumab mertansine administered intravenously in female patients with CD44v6 positive metastatic breast cancer with repeated administration in patients with clinical benefit.
|
||||
Cantuzumab mertansine [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
0.00%
|
|||
| Patients Enrolled |
Histological documentation of advanced or metastatic epithelial solid tumor which were likely to express the CanAg antigen, that were refractory or resistant to standard chemotherapy, or for which no effective standard therapy exists.
|
||||
| Administration Dosage |
IV infusion at an initial dose of 30 mg/m2, three times per week for three consecutive weeks for a total of nine doses.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [9] | ||||
| Patients Enrolled |
Solid malignancies refractory to standard therapy or for whom no standard therapy existed.
|
||||
| Administration Dosage |
IV cantuzumab mertansine was administered at a rate of 1 mg/min for 30 minutes and then increased to 3 mg/min if hypersensitivity phenomena were not observed. Treatment courses were repeated every 3 weeks.
|
||||
F105-SPP-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 17.58% (Day 49) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
The rear flank region of female athymic rats were implanted with 5x106 cells subcutaneously (0.2 ml of25 x106 cells/ml) on the rear flank area. When mean tumor volumes reached to 250 mm3, dosed intravenously (15 mg/kg) on days 17 and 29 after tumor cell injection.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 20.88% (Day 37) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Female athymic rats were inoculated with 5x106 HT29 cells subcutancously on the rear flank area. The animals were stratified into 13 groups, 6 animals per group based on a mean tumor volume for each group of approximately 250 mm3. On the day of grouping (day 7) each group received its initial dosing (175 ug/kg).
|
||||
| In Vivo Model | HT-29 CDX model | ||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
CNTO95-SPP-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 28.74% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 3 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 44.54% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 6 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.81% (Day 37) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Female athymic rats were inoculated with 5x106 HT29 cells subcutancously on the rear flank area. The animals were stratified into 13 groups, 6 animals per group based on a mean tumor volume for each group of approximately 250 mm3. On the day of grouping (day 7) each group received its initial dosing (175 ug/kg).
|
||||
| In Vivo Model | HT-29 CDX model | ||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 4 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 71.21% (Day 24) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Nine-week-old athymic nude rats were subcutaneously inoculated with A375.S2human melanoma cells. ADC (5 mg/kg) andappropriate control compounds were intravenously injected (three injection every other day inthe first week followed by one injection per week for two weeks.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.15% (Day 24) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Nine-week-old athymic nude rats were subcutaneously inoculated with A375.S2human melanoma cells. ADC (10 mg/kg) andappropriate control compounds were intravenously injected (three injection every other day inthe first week followed by one injection per week for two weeks.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.23% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 10 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.10% (Day 49) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
The rear flank region of female athymic rats were implanted with 5x106 cells subcutaneously (0.2 ml of25 x106 cells/ml) on the rear flank area. When mean tumor volumes reached to 250 mm3, dosed intravenously (15 mg/kg) on days 17 and 29 after tumor cell injection.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.81% (Day 35) | Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
On the day of grouping (Day 0), animals were weighed and intravenously injectedwith control ADC at 25 mg/kg. All testand control articles were given in a volume of 1ml/100 gm of body weight. CNTO 364 at 15 mg/kg groups was administered i.v. on a q7dx5 schedule.
|
||||
| In Vivo Model | A375.S2 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.00 ug/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were seeded into white 96-well tissue culture plates (5000 cells/well)in culture medium and incubated for 16 hrs. Serial dilutions of immunoconjugates were added toeach appropraite wells (0-20 ug/ml). Tissue culture plates were incubated at 37°C for 96 hrs.
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.20 ug/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were seeded into white 96-well tissue culture plates (5000 cells/well)in culture medium and incubated for 16 hrs. Serial dilutions of immunoconjugates were added toeach appropraite wells (0-20 ug/ml). Tissue culture plates were incubated at 37°C for 96 hrs.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.19 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 4 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.27 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.27 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | A2780 cells | CVCL_0134 | ||
Mirvetuximab-SPP-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
32.17% (Day 30)
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Animals with established tumors of about 130 mm3 were treated with intravenous single injection of the M9346A-DM conjugates at 2.5±0.2 mg/kg, equivalent to 51±3 ug conjugated maytansinoid per kg The conjugates were injected on day 20 after cell inoculation.
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
50.00% (Day 32)
|
High FOLR1 expression (FOLR1+++; 1,300,000 FOLR1 molecules/cell) | ||
| Method Description |
Animals with established tumors of about 130 mm3 were treated with intravenous single injection of the M9346A-DM conjugates at 2.5±0.2 mg/kg, equivalent to 51±3 ug conjugated maytansinoid per kg The conjugates were injected on day 7 after cell inoculation.
|
||||
| In Vivo Model | Ovarian carcinoma Igrov-1 CDX model | ||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.11±0.04 nM
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.13±0.08 nM
|
Moderate FOLR1 expression (FOLR1++; 290,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Gestational choriocarcinoma | JEG-3 cells | CVCL_0363 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.14±0.05 nM
|
High FOLR1 expression (FOLR1+++; 1,300,000 FOLR1 molecules/cell) | ||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
Anti-KIT NEG085?SPP-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 41.55% (Day 27) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (2.5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.55% (Day 27) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (5 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 91.32% (Day 27) | Positive KIT expression (KIT+++/++) | ||
| Method Description |
Female SCID-beige mice were implanted subcutaneously with 10,000,000 NCI-H1048 cells. The total injection volume containingcells in suspension was 200 ul. Mice were enrolled in the study 15 days post implantation withaverage tumor volume of about 120 mm3. All treated groups received a single intravenous dose of 2 mg/kg. After being randomly assigned to groups (n = 8/group), mice were administered a single i.v.dose of TBS (5 ml/kg) or ADC (10 mg/kg).
Click to Show/Hide
|
||||
| In Vivo Model | NCI-H1048 CDX model | ||||
| In Vitro Model | Lung small cell carcinoma | NCI-H1048 cells | CVCL_1453 | ||
B4-SPP-DC44 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 64.00% (Day 9) | Positive CD19 expression (CD19+++/++) | ||
| Method Description |
Animals with established subcutaneous xenograft Ramos tumors were treated with either huB4-SPP-DC4 (DC4 dose of 75 ug/kg, qdx5), or huB4-SPP-DC44 (DC44 dose of 75 ug/kg, qdx5), unconjugated DC4 (75 ug/kg, qdx5), unconjugated DC44 (75 ug/kg, qdx5), or with phosphate-buffered saline vehicle (control), administered intravenously, and the tumor growth was monitored.
Click to Show/Hide
|
||||
| In Vivo Model | Ramos CDX model | ||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.40 nM
|
Positive CD19 expression (CD19+++/++) | ||
| Method Description |
Cytotoxicity of B4-SPP-DCx Conjugates (with acid phosphatase treatment) against Ramos (Ag+) and HL60/s (Ag-) Cells.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 1.80 nM | Negative CD19 expression(CD19-) | ||
| Method Description |
Cytotoxicity of B4-SPP-DCx Conjugates (with acid phosphatase treatment) against Ramos (Ag+) and HL60/s (Ag-) Cells.
|
||||
| In Vitro Model | Adult acute myeloid leukemia | HL-60 cells | CVCL_0002 | ||
B-B4-SPP-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.45% (Day 20) | Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
MOLP-8 cells (1.5x107 cells per mouse) suspended in a 50:50 mixture of serum free media and matrigel were injected subcutaneously in the area under the right shoulder in 100 ul. Nine groups (n=6) were treated with a single intravenous injection of ADCs, each at doses of 250 ug/kg.
|
||||
| In Vivo Model | MOLP-8 CDX model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
Tmab-SPP-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.60% (Day 10) | High HER2 expression (HER2+++) | ||
| Method Description |
Mice bearing mammary tumor transplants from the MMTV-HER2 Fo5 line were given a single iv injection (10 mg/kg) of Tmab-SPP-DM1, Tmab-SSNPP-DM3, Tmab-SSNPP-DM4, Tmab-MCC-DM1, or vehicle (n=7 mice per group), and tumor growth was monitored for 25 days.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.22 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. For measurement of apoptosis, BT-474 and SK-BR-3 were exposed to trastuzumab or trastuzumab-DM for 48 h.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.26 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. For measurement of apoptosis, BT-474 and SK-BR-3 were exposed to trastuzumab or trastuzumab-DM for 48 h.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
41.37 nM
|
Negative HER2 expression (HER2-) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100.00 nM | Low HER2 expression (HER2+) | ||
| Method Description |
The effects of trastuzumab and trastuzumab-maytansinoid conjugates on tumor cell viability were assessed using Cell Titer-Glo. Cells were plated in black-walled 96-well plates (20,000 per well for BT-474; 10,000 cells per well for all other lines) and allowed to adhere overnight at 37°C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh culture medium containing different concentrations of trastuzumab, trastuzumab ADC, or free DM1, and the cells incubated for varying periods of time.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
nBT062-SPP-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.61% (Day 30) | Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
MOLP-8 cells (1.5x107 cells per mouse) suspended in a 50:50 mixture of serum free media and matrigel were injected subcutaneously in the area under the right shoulder in 100 ul. Nine groups (n=6) were treated with a single intravenous injection of ADCs, each at doses of 250 ug/kg.
|
||||
| In Vivo Model | MOLP-8 CDX model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 84.27% (Day 20) | Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
MOLP-8 cells (1.5x107 cells per mouse) suspended in a 50:50 mixture of serum free media and matrigel were injected subcutaneously in the area under the right shoulder in 100 ul. Nine groups (n=6) were treated with a single intravenous injection of ADCs, each at doses of 250 ug/kg.
|
||||
| In Vivo Model | MOLP-8 CDX model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 92.11% (Day 30) | Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
MOLP-8 cells (1.5x107 cells per mouse) suspended in a 50:50 mixture of serum free media and matrigel were injected subcutaneously in the area under the right shoulder in 100 ul. Nine groups (n=6) were treated with a single intravenous injection of ADCs, each at doses of 450 ug/kg.
|
||||
| In Vivo Model | MOLP-8 CDX model | ||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00 nM-10.00 nM
|
Positive CD138 expression (CD138 +++/++) | ||
| Method Description |
CD138+ MOLP-8 cells were seeded in flat bottom plates at 3000 cells/well. CD138- BJAB control cells were seeded at 1000 cells/weli. The cells were treated with nBT062-SPDB-DM4nBT062-SPP-DM1 or nBT062-SMCC-DM1 at different concentrations for five days.
|
||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Negative CD138 expression (CD138 -) | ||
| Method Description |
CD138+ MOLP-8 cells were seeded in flat bottom plates at 3000 cells/well. CD138- BJAB control cells were seeded at 1000 cells/weli. The cells were treated with nBT062-SPDB-DM4nBT062-SPP-DM1 or nBT062-SMCC-DM1 at different concentrations for five days.
|
||||
| In Vitro Model | Burkitt lymphoma | BJAB cells | CVCL_5711 | ||
B4-SPP-DC4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 84.00% (Day 9) | Positive CD19 expression (CD19+++/++) | ||
| Method Description |
Animals with established subcutaneous xenograft Ramos tumors were treated with either huB4-SPP-DC4 (DC4 dose of 75 ug/kg, qdx5), or huB4-SPP-DC44 (DC44 dose of 75 ug/kg, qdx5), unconjugated DC4 (75 ug/kg, qdx5), unconjugated DC44 (75 ug/kg, qdx5), or with phosphate-buffered saline vehicle (control), administered intravenously, and the tumor growth was monitored.
Click to Show/Hide
|
||||
| In Vivo Model | Ramos CDX model | ||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
11.00 pM
|
Positive CD19 expression (CD19+++/++) | ||
| Method Description |
Cytotoxicity of B4-SPP-DCx Conjugates (without acid phosphatase treatment) against Ramos (Ag+) and HL60/s (Ag-) Cells.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.74 nM
|
Positive CD19 expression (CD19+++/++) | ||
| Method Description |
Cytotoxicity of B4-SPP-DCx Conjugates (with acid phosphatase treatment) against Ramos (Ag+) and HL60/s (Ag-) Cells.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 1.50 nM | Negative CD19 expression(CD19-) | ||
| Method Description |
Cytotoxicity of B4-SPP-DCx Conjugates (with acid phosphatase treatment) against Ramos (Ag+) and HL60/s (Ag-) Cells.
|
||||
| In Vitro Model | Adult acute myeloid leukemia | HL-60 cells | CVCL_0002 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Negative CD19 expression(CD19-) | ||
| Method Description |
Cytotoxicity of B4-SPP-DCx Conjugates (without acid phosphatase treatment) against Ramos (Ag+) and HL60/s (Ag-) Cells.
|
||||
| In Vitro Model | Adult acute myeloid leukemia | HL-60 cells | CVCL_0002 | ||
huMov19-SPP-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.72% (Day 17) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 2.5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.09% (Day 17) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.20 nM
|
Moderate FOLR1 expression (FOLR1 ++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Gestational choriocarcinoma | JEG-3 cells | CVCL_0363 | ||
M9346A-SPP-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.98% (Day 20) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OVCAR-3 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
muDS6-DM1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.50% (Day 30) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
To demonstrate the in vivo activity of the muDS6-DM1 conjugate,human tumor xenografts were established in SCID mice.A subcutaneous model of the human pancreatic cancer cell-line HPAC was developed. The dose was 27.7 mg/kg qw x2.
|
||||
| In Vivo Model | HPAC CDX model | ||||
| In Vitro Model | Pancreatic adenocarcinoma | HPAC cells | CVCL_3517 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 30) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
To demonstrate the in vivo activity of the muDS6-DM1 conjugate,human tumor xenografts were established in SCID mice.A subcutaneous model of the human cervical cancer cell-line HeLa was developed. The dose was 27.7 mg/kg qw x2.
|
||||
| In Vivo Model | HeLa CDX model | ||||
| In Vitro Model | Endocervical adenocarcinoma | HeLa cells | CVCL_0030 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 30) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
To demonstrate the in vivo activity of the muDS6-DM1 conjugate,human tumor xenografts were established in SCID mice.A subcutaneous model of the human ovarian cancer cell-line TOV-21G was developed. The dose was 27.7 mg/kg qw x2.
|
||||
| In Vivo Model | TOV-21G CDX model | ||||
| In Vitro Model | Ovarian clear cell adenocarcinoma | TOV-21G cells | CVCL_3613 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 30) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
To demonstrate the in vivo activity of the muDS6-DM1 conjugate,human tumor xenografts were established inSCID mice.A subcutaneous model of the human cervicalcarcinoma cell-line KB was developed. The dose was 150 ug/kg every day for 5 days.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 30) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
To demonstrate the in vivo activity of the muDS6-DM1 conjugate,human tumor xenografts were established inSCID mice.A subcutaneous model of the human cervicalcarcinoma cell-line KB was developed. The dose was 250 ug/kg every day for 5 days.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 30) | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
To demonstrate the in vivo activity of the muDS6-DM1 conjugate,human tumor xenografts were established inSCID mice.A subcutaneous model of the human cervicalcarcinoma cell-line KB was developed. The dose was 27.7 mg/kg qw x2.
|
||||
| In Vivo Model | OVCAR-5 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-5 cells | CVCL_1628 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Ductal carcinoma | BT-483 cells | CVCL_2319 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.45 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Invasive breast carcinoma | ZR-75-1 cells | CVCL_0588 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.46 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Endocervical adenocarcinoma | WISH cells | CVCL_1909 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.67 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Endocervical adenocarcinoma | WISH cells | CVCL_1909 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.80 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | Caov-3 cells | CVCL_0201 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.40 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.61 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | Caov-3 cells | CVCL_0201 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.80 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Endocervical adenocarcinoma | HeLa cells | CVCL_0030 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.80 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Pancreatic adenocarcinoma | HPAC cells | CVCL_3517 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.84 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Pancreatic adenocarcinoma | HPAC cells | CVCL_3517 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.00 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian clear cell adenocarcinoma | TOV-21G cells | CVCL_3613 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-20 cells | CVCL_0178 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | High grade ovarian serous adenocarcinoma | Caov-4 cells | CVCL_0202 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Pancreatic adenocarcinoma | Hs 766T cells | CVCL_0334 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-5 cells | CVCL_1628 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | T-47D cells | CVCL_0553 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 3.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
Clonogenic assays were conducted where cells (1000-2500 cells/well) were plated on6-well plates in 2 ml of conjugate diluted in culture media.The cells were continuously exposed to the conjugate at concentrations,generally between 3x10-11M toseveral 3x10-9 M,and were incubated in a 37°C,6% CO2, humidified chamber for 5-9 days.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | ZR-75-1 cells | CVCL_0588 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.01 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 20 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
6.88 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Ovarian clear cell adenocarcinoma | TOV-21G cells | CVCL_3613 | ||
| Experiment 21 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
10.00 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
| Experiment 22 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
14.40 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-20 cells | CVCL_0178 | ||
| Experiment 23 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Ductal carcinoma | BT-483 cells | CVCL_2319 | ||
| Experiment 24 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | High grade ovarian serous adenocarcinoma | Caov-4 cells | CVCL_0202 | ||
| Experiment 25 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Endocervical adenocarcinoma | HeLa cells | CVCL_0030 | ||
| Experiment 26 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 nM | Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Invasive breast carcinoma | T-47D cells | CVCL_0553 | ||
| Experiment 27 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
32.00 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Pancreatic adenocarcinoma | Hs 766T cells | CVCL_0334 | ||
| Experiment 28 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
846 nM
|
Positive CA6 expression (CA6 +++/++) | ||
| Method Description |
In the MTT assay, cells were seeded in 96-well plates ata density of 1000-5000 cells/well. The cells were plated with serial dilutions of either naked muDS6 or muDS6-DM1 immunoconjugate in 200 pl of culture media. The cells and antibody/conjugate mixtures were then incubated for 2-7 d, at which time cellviability was assessed by an MTT assay.
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-5 cells | CVCL_1628 | ||
B4-SPP-DC1 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.40 pM
|
Positive CD19 expression (CD19+++/++) | ||
| Method Description |
Cytotoxicity of B4-SPP-DCx Conjugates (without acid phosphatase treatment) against Ramos (Ag+) and HL60/s (Ag-) Cells.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.30 nM
|
Negative CD19 expression(CD19-) | ||
| Method Description |
Cytotoxicity of B4-SPP-DCx Conjugates (without acid phosphatase treatment) against Ramos (Ag+) and HL60/s (Ag-) Cells.
|
||||
| In Vitro Model | Adult acute myeloid leukemia | HL-60 cells | CVCL_0002 | ||
CNTO95-SPP-DM4 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.00 ug/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were seeded into white 96-well tissue culture plates (5000 cells/well)in culture medium and incubated for 16 hrs. Serial dilutions of immunoconjugates were added toeach appropraite wells (0-20 ug/ml). Tissue culture plates were incubated at 37°C for 96 hrs.
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.50 ug/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were seeded into white 96-well tissue culture plates (5000 cells/well)in culture medium and incubated for 16 hrs. Serial dilutions of immunoconjugates were added toeach appropraite wells (0-20 ug/ml). Tissue culture plates were incubated at 37°C for 96 hrs.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.29 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 4 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.30 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | A2780 cells | CVCL_0134 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.42 mg/mL
|
Positive ITGAV expression (ITGAV +++/++) | ||
| Method Description |
Cells were harvested, rinsedsuspended in serum free DMEM, and sequentially incubated for 60 minutes on ice with serialdiluted CNTO 95,CNTO 364, CNTO 365 and CNTO 366 and FITC-labeled anti-humanantibody (10 mg/ml).
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
References
