Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0YWAHM
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
DS-6000
|
|||||
| Synonyms |
DS 6000; DS-6000; DS-6000a; DS6000; Raludotatug deruxtecan
Click to Show/Hide
|
|||||
| Organization |
Daiichi Sankyo Co., Ltd.
|
|||||
| Drug Status |
Phase 2/3
|
|||||
| Indication |
In total 6 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
8
|
|||||
| Structure |
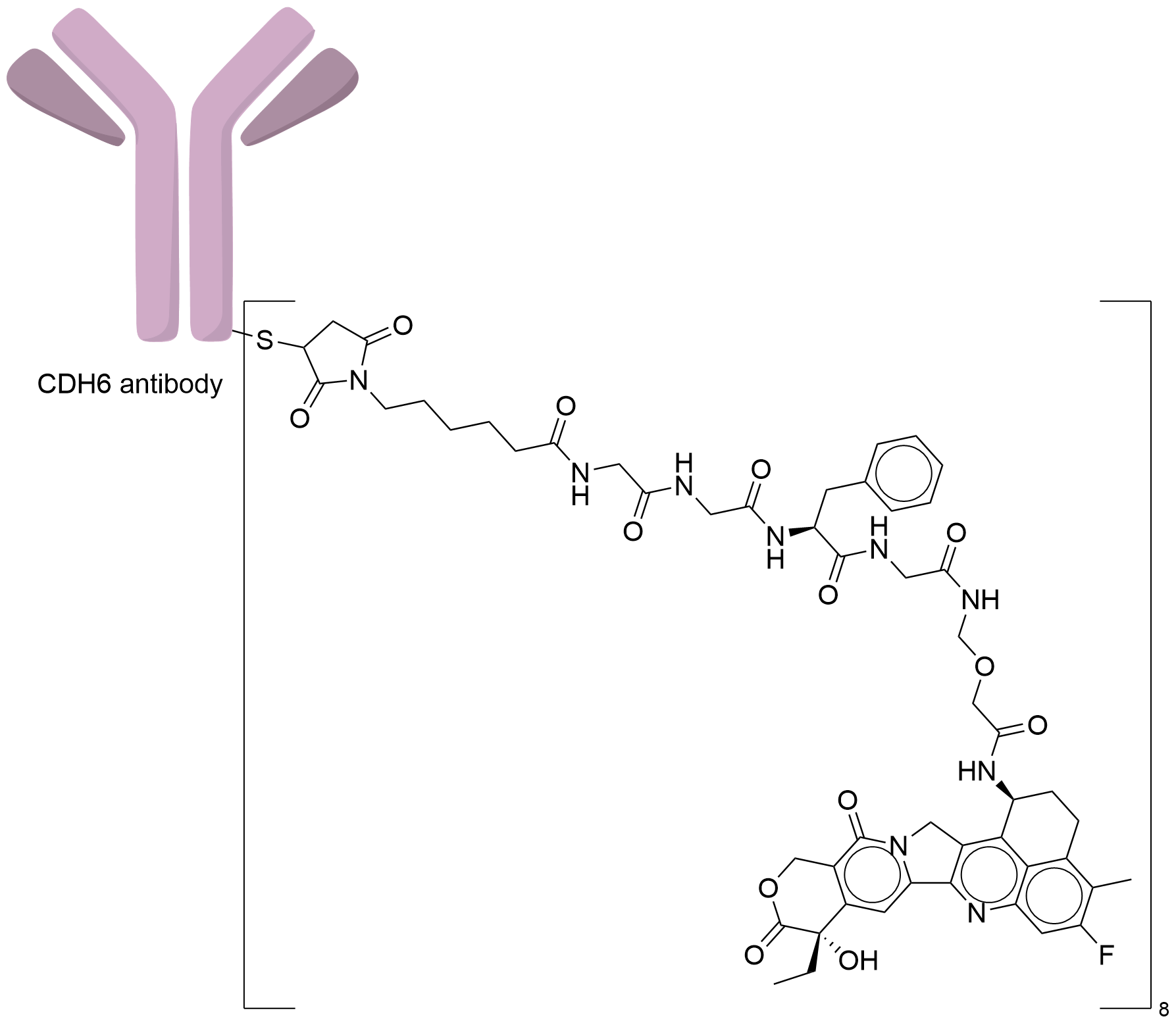
|
|||||
| Antibody Name |
Raludotatug
|
Antibody Info | ||||
| Antigen Name |
Cadherin-6 (CDH6)
|
Antigen Info | ||||
| Payload Name |
DX-8951 derivative (DXd)
|
Payload Info | ||||
| Therapeutic Target |
DNA topoisomerase 1 (TOP1)
|
Target Info | ||||
| Linker Name |
Mc-Gly-Gly-Phe-Gly
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
deruxtecan
|
|||||
| Puchem SID | ||||||
General Information of The Activity Data Related to This ADC
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
13.30% (all)
|
|||
| Patients Enrolled |
Patients with advanced renal cell carcinoma or ovarian cancer. Patients had received a median of 4 prior systemic therapy.
|
||||
| Administration Dosage |
First dose was 1.60 mg/kg followed by 3.20, 4.80, 6.40, 8.00, and 9.60 mg/kg every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT04707248 | Clinical Status | Phase 1 | ||
| Clinical Description | Phase 1, two-part, multi-center, first-in-human study of DS-6000A in subjects with advanced renal cell carcinoma and ovarian tumors. | ||||
References
