Payload Information
General Information of This Payload
| Payload ID | PAY0WDZAK |
|||||
|---|---|---|---|---|---|---|
| Name | Auristatin 0101 |
|||||
| Synonyms |
PF-06380101; 1436391-86-4; Auristatin 0101; Aur0101; Q8020AX34E; AUR-0101; 2-Methyl-L-Alanyl-N-[(3r,4s,5s)-3-Methoxy-1-{(2s)-2-[(1r,2r)-1-Methoxy-2-Methyl-3-Oxo-3-{[(1s)-2-Phenyl-1-(1,3-Thiazol-2-Yl)ethyl]amino}propyl]pyrrolidin-1-Yl}-5-Methyl-1-Oxoheptan-4-Yl]-N-Methyl-L-Valinamide; (2S)-2-((2-Amino-2-methyl-propanoyl)amino)-N-((1S,2R)-2-methoxy-4-((2S)-2-((1R,2R)-1-methoxy-2-methyl-3-oxo-3-(((1S)-2-phenyl-1-thiazol-2-yl-ethyl)amino)propyl)pyrrolidin-1-yl)-1-((1S)-1-methylpropyl)-4-oxo-butyl)-N,3-dimethyl-butanamide; (S)-2-(2-amino-2-methylpropanamido)-N-((3R,4S,5S)-3-methoxy-1-((S)-2-((1R,2R)-1-methoxy-2-methyl-3-oxo-3-(((S)-2-phenyl-1-(thiazol-2-yl)ethyl)amino)propyl)pyrrolidin-1-yl)-5-methyl-1-oxoheptan-4-yl)-N,3-dimethylbutanamide; L-Valinamide, 2-methylalanyl-N-((1S,2R)-2-methoxy-4-((2S)-2-((1R,2R)-1-methoxy-2-methyl-3-oxo-3-(((1S)-2-phenyl-1-(2-thiazolyl)ethyl)amino)propyl)-1-pyrrolidinyl)-1-((1S)-1-methylpropyl)-4-oxobutyl)-N-methyl-; Auristatin-0101; UNII-Q8020AX34E; CHEMBL3359827; SCHEMBL14936149; AKOS040742399; CS-3706; NCGC00485972-01; HY-12522; MS-31297; F85106; Q27454197; (2S)-2-[(2-amino-2-methylpropanoyl)amino]-N-[(3R,4S,5S)-3-methoxy-1-[(2S)-2-[(1R,2R)-1-methoxy-2-methyl-3-oxo-3-[[(1S)-2-phenyl-1-(1,3-thiazol-2-yl)ethyl]amino]propyl]pyrrolidin-1-yl]-5-methyl-1-oxoheptan-4-yl]-N,3-dimethylbutanamide; 2-methylalanyl-N-[(3R,4S,5S)-3-methoxy-1-{(2S)-2-[(1R,2R)-1-methoxy-2-methyl-3-oxo-3-{[(1S)-2-phenyl-1-(1,3-thiazol-2-yl)ethyl]amino}propyl]pyrrolidin-1-yl}-5-methyl-1-oxoheptan-4-yl]-N-methyl-L-valinamide; 3WD
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
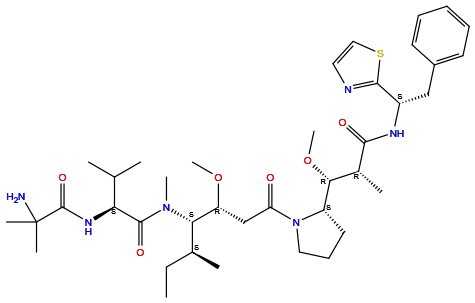
|
|||||
| Formula | C39H62N6O6S |
|||||
| Isosmiles | [H]c1nc([C@@]([H])(N([H])C(=O)[C@]([H])(C([H])([H])[H])[C@@]([H])(OC([H])([H])[H])[C@@]2([H])N(C(=O)C([H])([H])[C@@]([H])(OC([H])([H])[H])[C@@]([H])(N(C(=O)[C@@]([H])(N([H])C(=O)C(N([H])[H])(C([H])([H])[H])C([H])([H])[H])C([H])(C([H])([H])[H])C([H])([H])[H])C([H])([H])[H])[C@@]([H])(C([H])([H])[H])C([H])([H])C([H])([H])[H])C([H])([H])C([H])([H])C2([H])[H])C([H])([H])c2c([H])c([H])c([H])c([H])c2[H])sc1[H] |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C39H62N6O6S/c1-11-25(4)33(44(8)37(48)32(24(2)3)43-38(49)39(6,7)40)30(50-9)23-31(46)45-20-15-18-29(45)34(51-10)26(5)35(47)42-28(36-41-19-21-52-36)22-27-16-13-12-14-17-27/h12-14,16-17,19,21,24-26,28-30,32-34H,11,15,18,20,22-23,40H2,1-10H3,(H,42,47)(H,43,49)/t25-,26+,28-,29-,30+,32-,33-,34+/m0/s1
|
|||||
| InChIKey |
QAAFNSMAIAVCHE-BZLYQNAUSA-N
|
|||||
| IUPAC Name |
(2S)-2-[(2-amino-2-methylpropanoyl)amino]-N-[(3R,4S,5S)-3-methoxy-1-[(2S)-2-[(1R,2R)-1-methoxy-2-methyl-3-oxo-3-[[(1S)-2-phenyl-1-(1,3-thiazol-2-yl)ethyl]amino]propyl]pyrrolidin-1-yl]-5-methyl-1-oxoheptan-4-yl]-N,3-dimethylbutanamide
|
|||||
| Pharmaceutical Properties | Molecule Weight |
743.028 |
Polar area |
156.19 |
||
Complexity |
742.4451547 |
xlogp Value |
4.3415 |
|||
Heavy Count |
52 |
Rot Bonds |
30 |
|||
Hbond acc |
9 |
Hbond Donor |
3 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) | 0.8 | nM |
HT-29 cells
|
Colon adenocarcinoma
|
[1] |
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
PYX-201 [Phase 1]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05720117 | Phase Status | Phase 1 | ||
| Clinical Description |
A first-in-human, open-label, multicenter, phase 1 clinical study to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics, and preliminary efficacy of PYX-201 in participants with advanced solid tumors.
|
||||
Cofetuzumab pelidotin [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Partial Response (PR) |
16.67%
|
|||
| Patients Enrolled |
Metastatic TNBC or ER low (ER and PgR <5%, HER2 negative) breast cancer, and had received at least one prior chemotherapy for metastatic disease.
|
||||
| Administration Dosage |
Gedatolisib 110 mg weekly + cofetuzumab pelidotin 1.4 mg/kg every 3 weeks, 180 mg + 1.4 mg/kg, and 180 mg + 2.8 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03243331 | Phase Status | Phase 1 | ||
| Clinical Description |
An initial safety study of gedatolisib plus PTK7-ADC for metastatic triple-negative breast cancer.
|
||||
| Primary Endpoint |
A total of 18 pts were enrolled in three dose cohorts: gedatolisib 110 mg weekly cofetuzumab pelidotin 1.40 mg/kg every 3 weeks (n=4), 180 mg, 1.40 mg/kg (n=3), and 180 mg, 2.80 mg/kg (n=11). Nausea, anorexia, fatigue, and mucositis were common but rarely reached grade 3 severity. Myelosuppression was uncommon. ORR was 16.67% (3/18). An additional 3 pts had stable disease (of these 2 had stable disease for>18 weeks); CB18 was 27.80%. Median PFS was 2.00 months (95% confidence interval for PFS: 1.20-6.20). Pts with clinical benefit were enriched with genomic alterations in the PI3K and PTK7 pathways.
Click to Show/Hide
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
27.00% (Ovarian cancer, every 3 weeks)
16.00% (NSCLC, every 3 weeks) 21.00% (TNBC, every 3 weeks) 26.00% (Ovarian cancer, every 2 weeks) 33.00% (NSCLC, every 2 weeks) |
|||
| Patients Enrolled |
Locally advanced or metastatic solid tumors resistant to standard therapy or with no available standard therapy, and Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1.
|
||||
| Administration Dosage |
Intravenously every 3 weeks at 0.20-3.70 mg/kg or every 2 weeks at 2.10-3.20 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02222922 | Phase Status | Phase 1 | ||
| Clinical Description |
A first-in-human phase 1, dose escalation, safety and pharmacokinetic study of PF-06647020 in adult patients with advanced solid tumors.
|
||||
| Primary Endpoint |
The most common, treatment-related adverse events for PF-06647020 administered every 3 weeks were nausea, alopecia,fatigue, headache, neutropenia, and vomiting (45%); 25% of patients had grade 3 neutropenia. Two patients experienced doselimiting toxicities (grade 3 headache and fatigue) at the highest every 3 weeks dose evaluated. The recommended phase II dose was 2.80 mg/kg every 3 weeks. The overall safety profile observed with PF-06647020 administered every 2 weeks was similar to that of the every 3 weeks regimen. Systemic exposure for the ADC and total antibody generally increased in a dose-proportional manner. Antitumor activity was observed in treated patients with overall objective response rates of 27.00% in ovarian cancer (n=63), 19% in NSCLC (n=31), and 21% in TNBC (n=29).
Click to Show/Hide
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [8] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04189614 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1b efficacy and safety study of cofetuzumab pelidotin (ABBV-647, a PTK7-targeting antibody drug conjugate) in subjects with PTK7-expressing, recurrent non-small cell lung cancer.
|
||||
PF-06664178 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
0.00%
|
|||
| Patients Enrolled |
Advanced solid tumors resistant to standard therapy, or for which no other therapy was available, and at least one measurable lesion defined by Response Evaluation Criteria in Solid Tumors (RECIST version 1.1).
|
||||
| Administration Dosage |
Once every 21 days as an intravenous infusion over approximately 60 min, doses starting from 0.15 mg/kg to 6.14 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02122146 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1, dose escalation study of Pf-06664178 in patients with locally advanced or metastatic solid tumors.
|
||||
| Primary Endpoint |
In the 29 response-evaluable patients,the best overall response observed was limited to stable disease (SD) in 11 patients(37.90%) with PR or CR.
|
||||
| Other Endpoint |
Doses explored ranged from 0.15 mg/kg to 4.80 mg/kg. Doses of 3.60 mg/kg,4.20 mg/kg and 4.80 mg/kg were considered intolerable due to DLTs. MTD and RP2D were not determined.
|
||||
PF-06650808 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
9.68% (all response-evaluable patients)
16.67% (patients with breast cancer) 21.43% (patients with ER+ breast cancer) |
|||
| Patients Enrolled |
Locally advanced/metastatic solid tumors resistant to standard therapy or with no available standard therapy; Eastern Cooperative Oncology Group (ECOG) performance score (PS) 01; a life expectancy 12 weeks; and adequate bone marrow, hepatic, and renal function.
|
||||
| Administration Dosage |
Intravenously every 3 weeks (Q3W) starting at a dose of 0.20 mg/kg to be escalated up to 6.40 mg/kg, following the modified continual reassessment method (mCRM).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02129205 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 dose escalation study evaluating the safety and tolerability of PF-06650808 In patients with advanced solid tumors.
|
||||
| Primary Endpoint |
OrR=9.68% for all response-evaluable patients (N=3/31), ORR=16.67% for patients with breast cancer (N=3/18), ORR=21.43% for patients with ER+ breast cancer (N=3/14). Five patients with advanced BC achieved PR as best overall response (BOR): 2 at 2.0 mg/kg, 1 at 2.4 mg/kg, and 2 at 3.6 mg/kg.
|
||||
| Other Endpoint |
The maximum tolerated dose (MTD) was estimated to be 2.40 mg/kg.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [7] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02129205 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 dose escalation study evaluating the safety and tolerability of PF-06650808 in patients with advanced solid tumors.
|
||||
PF-06804103 [Phase 1 (Terminated)]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00%
|
High HER2 expression (HER2+++) | ||
| Method Description |
In cell line xenograft (CLX) studies, nude mice were injected subcutaneously in the flank with suspensions of 1 x106 NCI-N87, in 50% Matrigel (BD Biosciences). Mice were randomized into study groups when tumors reached approximately 150-300 mm3. Either PBS (Gibco, catalog no., 14190-144, as vehicle), PF-06804103, or PT-DM1 at different doses was administered intravenously starting on day 0 for a total of four doses, 4 days apart (four times every 4 days).
Click to Show/Hide
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00%
|
Moderate HER2 expression (HER2++) | ||
| Method Description |
In cell line xenograft (CLX) studies, nude mice were injected subcutaneously in the flank with suspensions of 10 x106 BT474 cells, in 50% Matrigel (BD Biosciences). Mice were randomized into study groups when tumors reached approximately 150-300 mm3. Either PBS (Gibco, catalog no., 14190-144, as vehicle), PF-06804103, or PT-DM1 at different doses was administered intravenously starting on day 0 for a total of four doses, 4 days apart (four times every 4 days).
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | BT474-M1 cells | CVCL_0179 | ||
EDB-ADC [Investigative]
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 14) | High FN1 expression (FN1+++) | ||
| Method Description |
EDB+FN expression was evaluated in NSCLC and pancreatic cancer xenografts in immunocompromised mice using IHC. Mice were treated intravenously every 4 days with phosphate-buffered saline (PBS),Neg-vc0101 (Neg-ADC),EDC-vc0101 (EDB-ADC). This tumor model was injected intravenously with EDB-ADC 0.75 mg/kg q4dx4.
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: PDX-PAX-13565) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 26.20% (Day 14) | High FN1 expression (FN1+++) | ||
| Method Description |
EDB+FN expression was evaluated in NSCLC and pancreatic cancer xenografts in immunocompromised mice using IHC. Mice were treated intravenously every 4 days with phosphate-buffered saline (PBS),Neg-vc0101 (Neg-ADC),EDC-vc0101 (EDB-ADC). This tumor model was injected intravenously with EDB-ADC 1.5 mg/kg q4dx4.
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: PDX-PAX-13565) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 43.20% (Day 28) | High FN1 expression (FN1+++) | ||
| Method Description |
EDB+FN expression was evaluated in NSCLC and pancreatic cancer xenografts in immunocompromised mice using IHC. Mice were treated intravenously every 4 days with phosphate-buffered saline (PBS),Neg-vc0101 (Neg-ADC),EDC-vc0101 (EDB-ADC). This tumor model was injected intravenously with EDB-ADC 0.3 mg/kg q4dx4.
|
||||
| In Vivo Model | Nonsmall cell lung cancer PDX model (PDX: PDX-NSX-11122) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 79.30% (Day 28) | High FN1 expression (FN1+++) | ||
| Method Description |
EDB+FN expression was evaluated in NSCLC and pancreatic cancer xenografts in immunocompromised mice using IHC. Mice were treated intravenously every 4 days with phosphate-buffered saline (PBS),Neg-vc0101 (Neg-ADC),EDC-vc0101 (EDB-ADC). This tumor model was injected intravenously with EDB-ADC 1 mg/kg q4dx4.
|
||||
| In Vivo Model | Nonsmall cell lung cancer PDX model (PDX: PDX-NSX-11122) | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.80% (Day 14) | High FN1 expression (FN1+++) | ||
| Method Description |
EDB+FN expression was evaluated in NSCLC and pancreatic cancer xenografts in immunocompromised mice using IHC. Mice were treated intravenously every 4 days with phosphate-buffered saline (PBS),Neg-vc0101 (Neg-ADC),EDC-vc0101 (EDB-ADC). This tumor model was injected intravenously with EDB-ADC 3 mg/kg q4dx4.
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: PDX-PAX-13565) | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.20% (Day 28) | High FN1 expression (FN1+++) | ||
| Method Description |
EDB+FN expression was evaluated in NSCLC and pancreatic cancer xenografts in immunocompromised mice using IHC. Mice were treated intravenously every 4 days with phosphate-buffered saline (PBS),Neg-vc0101 (Neg-ADC),EDC-vc0101 (EDB-ADC). This tumor model was injected intravenously with EDB-ADC 3 mg/kg q4dx4.
|
||||
| In Vivo Model | Nonsmall cell lung cancer PDX model (PDX: PDX-NSX-11122) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 67.00% (Day 15) | High FN1 expression (FN1+++) | ||
| Method Description |
EDB+FN expression was evaluated in NSCLC and pancreatic cancer xenografts in immunocompromised mice using IHC. Athymic nu/nu nude mice were injected subcutaneously in the right flank with 8 10 6 cells suspended in 100% Matrigel. This tumor model was injected intravenously with EDB-ADC 1 mg/kg q4dx4.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.10% (Day 15) | High FN1 expression (FN1+++) | ||
| Method Description |
EDB+FN expression was evaluated in NSCLC and pancreatic cancer xenografts in immunocompromised mice using IHC. Athymic nu/nu nude mice were injected subcutaneously in the right flank with 8 10 6 cells suspended in 100% Matrigel. This tumor model was injected intravenously with EDB-ADC 3 mg/kg q4dx4.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 30) | High FN1 expression (FN1+++) | ||
| Method Description |
EDB+FN expression was evaluated in NSCLC and pancreatic cancer xenografts in immunocompromised mice using IHC. Athymic nu/nu nude mice were injected subcutaneously in the right flank with 8 10 6 cells suspended in 100% Matrigel. This tumor model was injected intravenously with EDB-ADC 3 mg/kg q4dx4.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
Anti-IL13RA2-ADC [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 29.60% (Day 13) | High IL13RA2 expression (IL13RA2+++) | ||
| Method Description |
2 x106 A375 cells in 50% Cultrex basement membrane extract were injected subcutaneously in the flank of athymic nu/nu mice for FMT imaging or in the upper right shoulder for PET/CT imaging.
|
||||
| In Vivo Model | A375 xenograft model | ||||
| In Vitro Model | Amelanotic melanoma | A375 cells | CVCL_0132 | ||
3G10-TG6-vc0101 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 61.00% (Day 21) | Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Mice were treated with a single intravenous dose of the ADCs at 1.5 mg/kg.
|
||||
| In Vivo Model | Ramos CDX model | ||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 82.80% (Day 27) | Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Mice were treated with a single intravenous dose of the ADCs at 1.5 mg/kg.
|
||||
| In Vivo Model | HPB-ALL CDX model | ||||
| In Vitro Model | T acute lymphoblastic leukemia | HPB-ALL cells | CVCL_1820 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 92.90% (Day 21) | Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Mice were treated with a single intravenous dose of the ADCs at 3 mg/kg.
|
||||
| In Vivo Model | Ramos CDX model | ||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.00% (Day 27) | Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Mice were treated with a single intravenous dose of the ADCs at 3 mg/kg.
|
||||
| In Vivo Model | HPB-ALL CDX model | ||||
| In Vitro Model | T acute lymphoblastic leukemia | HPB-ALL cells | CVCL_1820 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.00% (Day 21) | Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Mice were treated with a single intravenous dose of the ADCs at 6 mg/kg.
|
||||
| In Vivo Model | HPB-ALL CDX model | ||||
| In Vitro Model | T acute lymphoblastic leukemia | HPB-ALL cells | CVCL_1820 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 21) | Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Mice were treated with a single intravenous dose of the ADCs at 6 mg/kg.
|
||||
| In Vivo Model | Ramos CDX model | ||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.05 nM
|
Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.06 nM
|
Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | HPB-ALL cells | CVCL_1820 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.06 nM
|
Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Adult T acute lymphoblastic leukemia | MOLT-4 cells | CVCL_0013 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.14 nM
|
Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Plasma cell myeloma | RPMI-8226 cells | CVCL_0014 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.86 nM
|
Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
8.53 nM
|
Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Plasma cell myeloma | MM1.S cells | CVCL_8792 | ||
6B6-TG6-vc0101 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 67.90% (Day 21) | Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Mice were treated with a single intravenous dose of the ADCs at 1.5 mg/kg.
|
||||
| In Vivo Model | Ramos CDX model | ||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 85.70% (Day 21) | Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Mice were treated with a single intravenous dose of the ADCs at 3 mg/kg.
|
||||
| In Vivo Model | Ramos CDX model | ||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.00% (Day 21) | Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Mice were treated with a single intravenous dose of the ADCs at 6 mg/kg.
|
||||
| In Vivo Model | Ramos CDX model | ||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.06 nM
|
Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Adult T acute lymphoblastic leukemia | MOLT-4 cells | CVCL_0013 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07 nM
|
Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Plasma cell myeloma | MOLP-8 cells | CVCL_2124 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07 nM
|
Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | HPB-ALL cells | CVCL_1820 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.25 nM
|
Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Plasma cell myeloma | RPMI-8226 cells | CVCL_0014 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.56 nM
|
Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Plasma cell myeloma | MM1.S cells | CVCL_8792 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.00 nM
|
Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
rcEDB-ADC [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 69.70% (Day 18) | High FN1 expression (FN1+++) | ||
| Method Description |
For studies using the mouse syngeneic cell line EMT6, 8-week-old Balb/c mice were injected in the left mammary fat pad with 1 x106 cells suspended in DPBS. Tumors were measured at least twice/week. This tumor model was injected intravenously with rcEDB-ADC 9 mg/kg single.
|
||||
| In Vivo Model | Breast cancer CDX model | ||||
| In Vitro Model | Mammary gland malignant neoplasms | EMT6 cells | CVCL_1923 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 69.70% (Day 18) | High FN1 expression (FN1+++) | ||
| Method Description |
For studies using the mouse syngeneic cell line EMT6, 8-week-old Balb/c mice were injected in the left mammary fat pad with 1 x106 cells suspended in DPBS. Tumors were measured at least twice/week. This tumor model was injected intravenously with rcEDB-ADC 3 mg/kg q4dx3.
|
||||
| In Vivo Model | Breast cancer CDX model | ||||
| In Vitro Model | Mammary gland malignant neoplasms | EMT6 cells | CVCL_1923 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 69.70% (Day 18) | High FN1 expression (FN1+++) | ||
| Method Description |
For studies using the mouse syngeneic cell line EMT6, 8-week-old Balb/c mice were injected in the left mammary fat pad with 1 x106 cells suspended in DPBS. Tumors were measured at least twice/week. This tumor model was injected intravenously with rcEDB-ADC 1.5 mg/kg q4dx3.
|
||||
| In Vivo Model | Breast cancer CDX model | ||||
| In Vitro Model | Mammary gland malignant neoplasms | EMT6 cells | CVCL_1923 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.30% (Day 18) | High FN1 expression (FN1+++) | ||
| Method Description |
For studies using the mouse syngeneic cell line EMT6, 8-week-old Balb/c mice were injected in the left mammary fat pad with 1 x106 cells suspended in DPBS. Tumors were measured at least twice/week. This tumor model was injected intravenously with rcEDB-ADC 4.5 mg/kg q4dx2.
|
||||
| In Vivo Model | Breast cancer CDX model | ||||
| In Vitro Model | Mammary gland malignant neoplasms | EMT6 cells | CVCL_1923 | ||
Anti-CXCR4 ADC 381 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.70 nM
|
High CXCR4 expression (CXCR4+++) | ||
| Method Description |
Cells were seeded in a culture-treated 96-well clear plate and incubated at 37°C under 5% CO2 for 24h. Serially diluted samples (50L) were added to each well and the plate was incubated at 37°C for 72h.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Anti-CXCR4 ADC 519 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.70 nM
|
High CXCR4 expression (CXCR4+++) | ||
| Method Description |
Cells were seeded in a culture-treated 96-well clear plate and incubated at 37°C under 5% CO2 for 24h. Serially diluted samples (50L) were added to each well and the plate was incubated at 37°C for 72h.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Anti-CXCR4 ADC 518 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.70 nM
|
High CXCR4 expression (CXCR4+++) | ||
| Method Description |
Cells were seeded in a culture-treated 96-well clear plate and incubated at 37°C under 5% CO2 for 24h. Serially diluted samples (50L) were added to each well and the plate was incubated at 37°C for 72h.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Anti-CXCR4 ADC 554 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.50 nM
|
High CXCR4 expression (CXCR4+++) | ||
| Method Description |
Cells were seeded in a culture-treated 96-well clear plate and incubated at 37°C under 5% CO2 for 24h. Serially diluted samples (50L) were added to each well and the plate was incubated at 37°C for 72h.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Anti-CXCR4 ADC 553 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
17.10 nM
|
High CXCR4 expression (CXCR4+++) | ||
| Method Description |
Cells were seeded in a culture-treated 96-well clear plate and incubated at 37°C under 5% CO2 for 24h. Serially diluted samples (50L) were added to each well and the plate was incubated at 37°C for 72h.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Anti-CXCR4 ADC 555 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
17.70 nM
|
High CXCR4 expression (CXCR4+++) | ||
| Method Description |
Cells were seeded in a culture-treated 96-well clear plate and incubated at 37°C under 5% CO2 for 24h. Serially diluted samples (50L) were added to each well and the plate was incubated at 37°C for 72h.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Anti-MSL1 mAb-Compound 31 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
37.38 nM
|
Positive MSL expression (MSL+++/++) | ||
| Method Description |
The cells were incubated in a CO2 incubat or with saturated water overnight, On the second day, two covalent thiol conjugated ADCs were serially diluted conjugates were added to the 96 well plate containing OVCAR3 cells, 100uL per well. The initial conjugate was 100000 ng/ml and diluted to 0.001 ng/ml. OVCAR3 cells with added ADC were incubated at 37 for 72 hours.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
Anti-CXCR4 ADC 556 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
39.70 nM
|
High CXCR4 expression (CXCR4+++) | ||
| Method Description |
Cells were seeded in a culture-treated 96-well clear plate and incubated at 37°C under 5% CO2 for 24h. Serially diluted samples (50L) were added to each well and the plate was incubated at 37°C for 72h.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
References
