Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0WRFYU
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
PF-06650808
|
|||||
| Synonyms |
PF 6650808; PF-06650808; PF-6650808
Click to Show/Hide
|
|||||
| Organization |
Pfizer Inc.
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
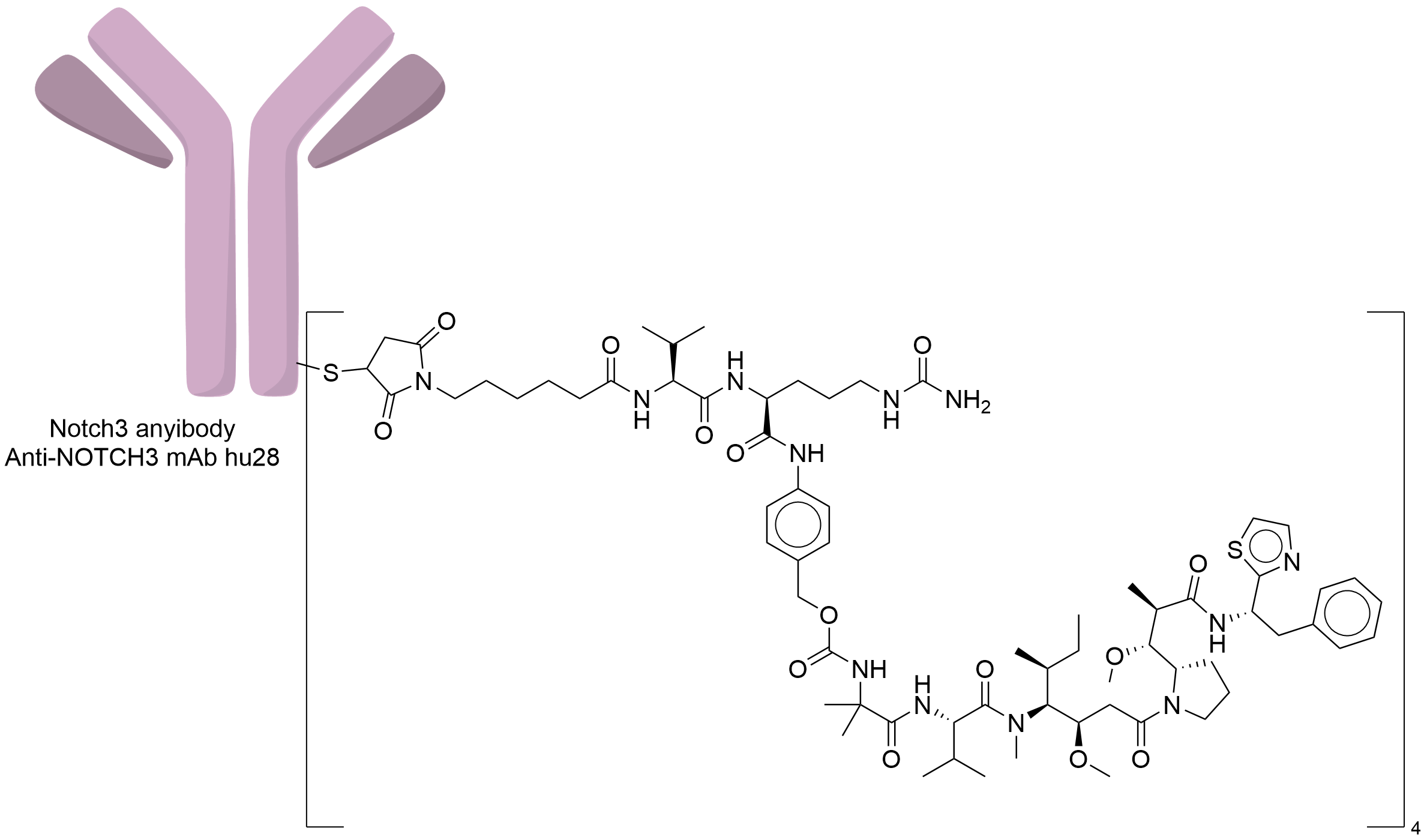
|
|||||
| Antibody Name |
Anti-NOTCH3 mAb hu28
|
Antibody Info | ||||
| Antigen Name |
Neurogenic locus notch homolog protein 3 (NOTCH3)
|
Antigen Info | ||||
| Payload Name |
Auristatin 0101
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
pelidotin
|
|||||
| TTD ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
9.68% (all response-evaluable patients)
16.67% (patients with breast cancer) 21.43% (patients with ER+ breast cancer) |
|||
| Patients Enrolled |
Locally advanced/metastatic solid tumors resistant to standard therapy or with no available standard therapy; Eastern Cooperative Oncology Group (ECOG) performance score (PS) 01; a life expectancy 12 weeks; and adequate bone marrow, hepatic, and renal function.
|
||||
| Administration Dosage |
Intravenously every 3 weeks (Q3W) starting at a dose of 0.20 mg/kg to be escalated up to 6.40 mg/kg, following the modified continual reassessment method (mCRM).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02129205 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 dose escalation study evaluating the safety and tolerability of PF-06650808 In patients with advanced solid tumors. | ||||
| Primary Endpoint |
OrR=9.68% for all response-evaluable patients (N=3/31), ORR=16.67% for patients with breast cancer (N=3/18), ORR=21.43% for patients with ER+ breast cancer (N=3/14). Five patients with advanced BC achieved PR as best overall response (BOR): 2 at 2.0 mg/kg, 1 at 2.4 mg/kg, and 2 at 3.6 mg/kg.
|
||||
| Other Endpoint |
The maximum tolerated dose (MTD) was estimated to be 2.40 mg/kg.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02129205 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 dose escalation study evaluating the safety and tolerability of PF-06650808 in patients with advanced solid tumors. | ||||
References
