Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0QCIPJ
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
rcEDB-ADC
|
|||||
| Synonyms |
RcEDB ADC; rcEDBADC
Click to Show/Hide
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 5 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3.4
|
|||||
| Structure |
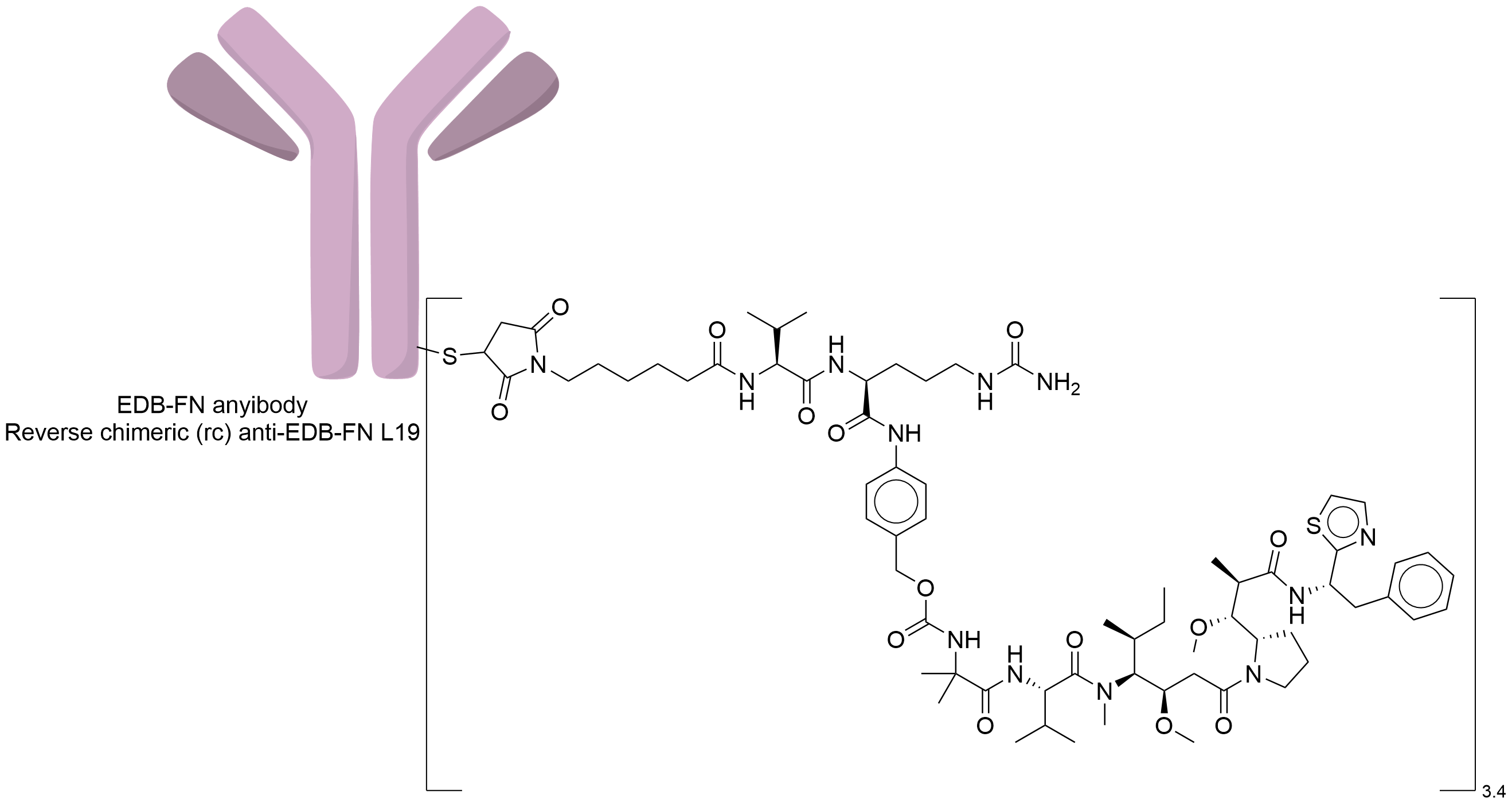
|
|||||
| Antibody Name |
Reverse chimeric (rc) Anti-EDB-FN L19
|
Antibody Info | ||||
| Antigen Name |
Fibronectin (FN1)
|
Antigen Info | ||||
| Payload Name |
Auristatin 0101
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Site-specific conjugated of maleimidocaproyl with cysteine free thiol via Michael addition.
|
|||||
| Combination Type |
Pelidotin
|
|||||
General Information of The Activity Data Related to This ADC
Discovered Using Cell Line-derived Xenograft Model
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 69.70% (Day 18) | High FN1 expression (FN1+++) | ||
| Method Description |
For studies using the mouse syngeneic cell line EMT6, 8-week-old Balb/c mice were injected in the left mammary fat pad with 1 x106 cells suspended in DPBS. Tumors were measured at least twice/week. This tumor model was injected intravenously with rcEDB-ADC 9 mg/kg single.
|
||||
| In Vivo Model | Breast cancer CDX model | ||||
| In Vitro Model | Mammary gland malignant neoplasms | EMT6 cells | CVCL_1923 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 69.70% (Day 18) | High FN1 expression (FN1+++) | ||
| Method Description |
For studies using the mouse syngeneic cell line EMT6, 8-week-old Balb/c mice were injected in the left mammary fat pad with 1 x106 cells suspended in DPBS. Tumors were measured at least twice/week. This tumor model was injected intravenously with rcEDB-ADC 3 mg/kg q4dx3.
|
||||
| In Vivo Model | Breast cancer CDX model | ||||
| In Vitro Model | Mammary gland malignant neoplasms | EMT6 cells | CVCL_1923 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 69.70% (Day 18) | High FN1 expression (FN1+++) | ||
| Method Description |
For studies using the mouse syngeneic cell line EMT6, 8-week-old Balb/c mice were injected in the left mammary fat pad with 1 x106 cells suspended in DPBS. Tumors were measured at least twice/week. This tumor model was injected intravenously with rcEDB-ADC 1.5 mg/kg q4dx3.
|
||||
| In Vivo Model | Breast cancer CDX model | ||||
| In Vitro Model | Mammary gland malignant neoplasms | EMT6 cells | CVCL_1923 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.30% (Day 18) | High FN1 expression (FN1+++) | ||
| Method Description |
For studies using the mouse syngeneic cell line EMT6, 8-week-old Balb/c mice were injected in the left mammary fat pad with 1 x106 cells suspended in DPBS. Tumors were measured at least twice/week. This tumor model was injected intravenously with rcEDB-ADC 4.5 mg/kg q4dx2.
|
||||
| In Vivo Model | Breast cancer CDX model | ||||
| In Vitro Model | Mammary gland malignant neoplasms | EMT6 cells | CVCL_1923 | ||
References
