Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0KTRKE
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
PF-06664178
|
|||||
| Synonyms |
PF 6664178; PF-06664178; PF06664178; PF6664178
Click to Show/Hide
|
|||||
| Organization |
Pfizer Inc.
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 4 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
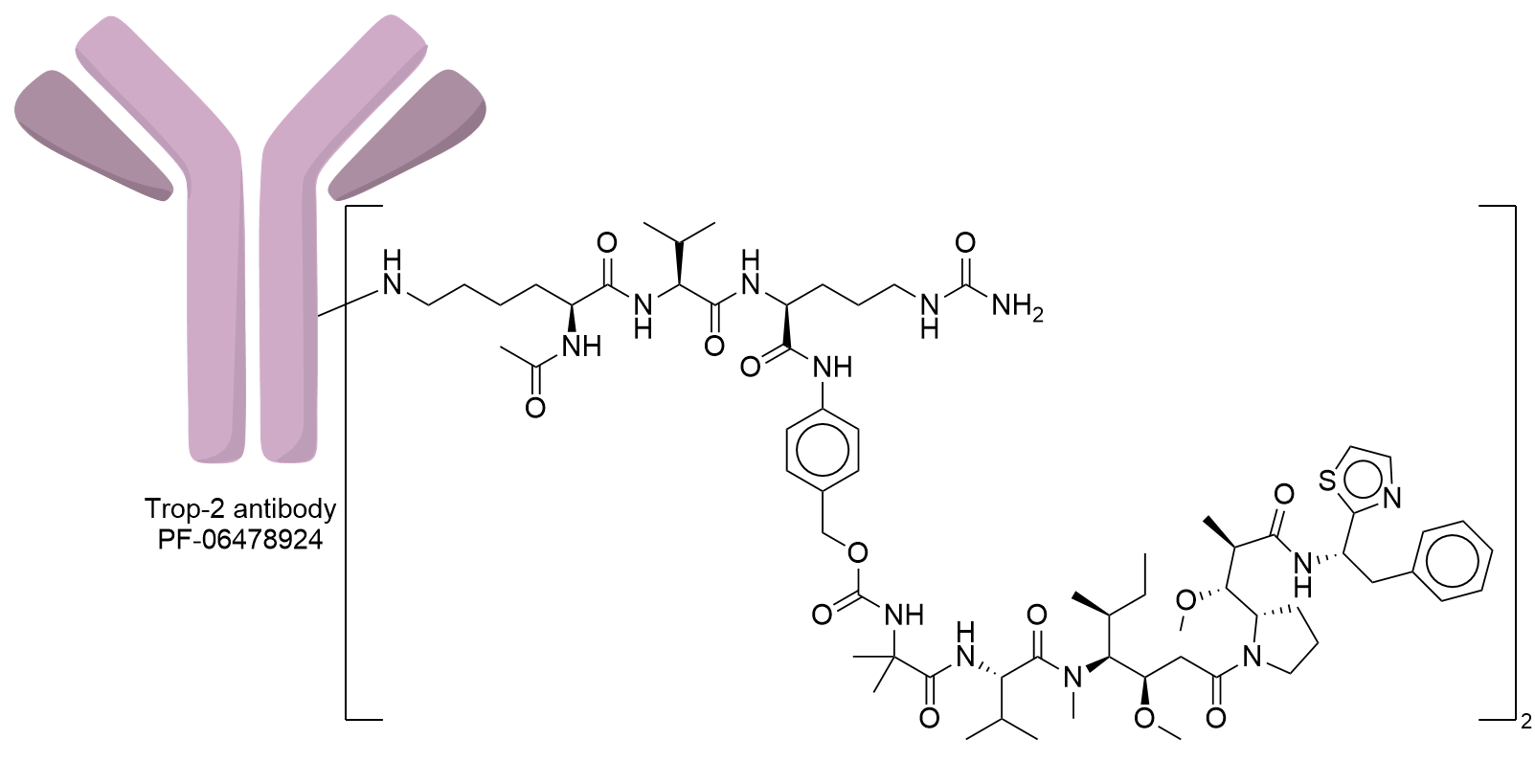
|
|||||
| Antibody Name |
PF-06478924
|
Antibody Info | ||||
| Antigen Name |
Tumor-associated calcium signal transducer 2 (TROP2)
|
Antigen Info | ||||
| Payload Name |
Auristatin 0101
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
AcLys-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| TTD ID | ||||||
General Information of The Activity Data Related to This ADC
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
0.00%
|
|||
| Patients Enrolled |
Advanced solid tumors resistant to standard therapy, or for which no other therapy was available, and at least one measurable lesion defined by Response Evaluation Criteria in Solid Tumors (RECIST version 1.1).
|
||||
| Administration Dosage |
Once every 21 days as an intravenous infusion over approximately 60 min, doses starting from 0.15 mg/kg to 6.14 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02122146 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, dose escalation study of Pf-06664178 in patients with locally advanced or metastatic solid tumors. | ||||
| Primary Endpoint |
In the 29 response-evaluable patients,the best overall response observed was limited to stable disease (SD) in 11 patients(37.90%) with PR or CR.
|
||||
| Other Endpoint |
Doses explored ranged from 0.15 mg/kg to 4.80 mg/kg. Doses of 3.60 mg/kg,4.20 mg/kg and 4.80 mg/kg were considered intolerable due to DLTs. MTD and RP2D were not determined.
|
||||
References
