Payload Information
General Information of This Payload
| Payload ID | PAY0SOSMQ |
|||||
|---|---|---|---|---|---|---|
| Name | seco-DUBA |
|||||
| Synonyms |
Seco-DUBA; Seco-duocarmycin; CYF7MUE6JF; 1227961-59-2; UNII-CYF7MUE6JF; Benzamide, N-(2-(((1S)-1-(chloromethyl)-1,2-dihydro-5-hydroxy-9-methyl-3H-benz(E)indol-3-yl)carbonyl)imidazo(1,2-a)pyridin-6-yl)-4-hydroxy-; N-(2-(((1S)-1-(Chloromethyl)-1,2-dihydro-5-hydroxy-9-methyl-3H-benz(E)indol-3-yl)carbonyl)imidazo(1,2-a)pyridin-6-yl)-4-hydroxybenzamide; SCHEMBL12289192; EX-A6014; AKOS040756558; HY-132180A; MS-29754; CS-0213565; F81356
Click to Show/Hide
|
|||||
| Target(s) | Human Deoxyribonucleic acid (hDNA) | |||||
| Structure |
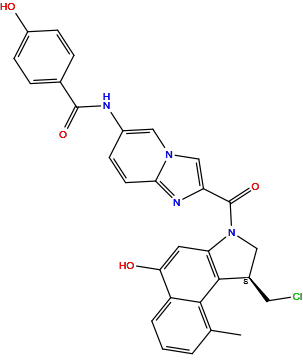
|
|||||
| Formula | C29H23ClN4O4 |
|||||
| Isosmiles | [H]Oc1c([H])c([H])c(C(=O)N([H])c2c([H])c([H])c3nc(C(=O)N4c5c([H])c(O[H])c6c([H])c([H])c([H])c(C([H])([H])[H])c6c5[C@]([H])(C([H])([H])Cl)C4([H])[H])c([H])n3c2[H])c([H])c1[H] |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C29H23ClN4O4/c1-16-3-2-4-21-24(36)11-23-27(26(16)21)18(12-30)13-34(23)29(38)22-15-33-14-19(7-10-25(33)32-22)31-28(37)17-5-8-20(35)9-6-17/h2-11,14-15,18,35-36H,12-13H2,1H3,(H,31,37)/t18-/m1/s1
|
|||||
| InChIKey |
VQAFBYLFRCCWNB-GOSISDBHSA-N
|
|||||
| IUPAC Name |
N-[2-[(1S)-1-(chloromethyl)-5-hydroxy-9-methyl-1,2-dihydrobenzo[e]indole-3-carbonyl]imidazo[1,2-a]pyridin-6-yl]-4-hydroxybenzamide
|
|||||
| Pharmaceutical Properties | Molecule Weight |
526.98 |
Polar area |
107.17 |
||
Complexity |
526.1407829 |
xlogp Value |
5.44222 |
|||
Heavy Count |
38 |
Rot Bonds |
7 |
|||
Hbond acc |
6 |
Hbond Donor |
3 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) | 0.09 | nM |
SK-BR-3 cells
|
Breast adenocarcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.09 | nM |
SW620 cells
|
Colon adenocarcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.43 | nM |
SK-OV-3 cells
|
Ovarian serous cystadenocarcinoma
|
[1] |
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
Trastuzumab duocarmazine [Phase 3]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03262935 | Phase Status | Phase 3 | ||
| Clinical Description |
A multi-centre, open-label, randomized clinical trial comparing the efficacy and safety of the antibody-drug conjugate SYD985 to physician's choice in patients with HER2-positive unresectable locally advanced or metastatic breast cancer.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04205630 | Phase Status | Phase 2 | ||
| Clinical Description |
A single-arm phase 2 trial to evaluate the safety and efficacy of the antibody-drug conjugate (ADC) SYD985 in patients with human epidermal growth factor receptor 2 (HER2)-expressing endometrial carcinoma who previously progressed on or after first line platinum-based chemotherapy.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT01042379 | Phase Status | Phase 2 | ||
| Clinical Description |
I-SPY trial (investigation of serial studies to predict your therapeutic response with imaging and molecular analysis 2).
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [5] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04983238 | Phase Status | Phase 1 | ||
| Clinical Description |
A multicenter, randomized, double-blind, placebo-controlled trial with a single arm run-in period to evaluate the safety and efficacy of sodium thiosulfate (BYON5667) Eye drops to reduce ocular toxicity in cancer patients treated with SYD985.
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [6] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04602117 | Phase Status | Phase 1 | ||
| Clinical Description |
ISPY-P1.01: evaluating the safety of weekly paclitaxel with trastuzumab duocarmazine (SYD985) in patients with metastatic cancer: a phase 1/Ib trial.
|
||||
| Experiment 6 Reporting the Activity Date of This ADC | [7] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04235101 | Phase Status | Phase 1 | ||
| Clinical Description |
A two-part phase 1 study with the antibody-drug conjugate SYD985 in combination with niraparib to evaluate safety, pharmacokinetics and efficacy in patients with HER2-expressing locally advanced or metastatic solid tumors.
|
||||
| Experiment 7 Reporting the Activity Date of This ADC | [8] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02277717 | Phase Status | Phase 1 | ||
| Clinical Description |
A two part first-in-human phase 1 study (with expanded cohorts) with the antibody-drug conjugate SYD985 to evaluate the safety, pharmacokinetics and efficacy in patients with locally advanced or metastatic solid tumors.
|
||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 33.30% (Day 76) | High HER2 expression (HER2+++; IHC 3+; FISH+) | ||
| Method Description |
All treatments were conducted at day 0 by a single dose, i.v, 3 mg/kg x1 injection into the tail vein, Data, depicted as mean tumour volume, consists of 6-8 animals per experimental group.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: MAXF-1162) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 42.90% (Day 80) | Low HER2 expression (HER2+; IHC +; FISH-) | ||
| Method Description |
All treatments were conducted at day 0 by a single dose, i.v, 3 mg/kg x1 injection into the tail vein, Data, depicted as mean tumour volume, consists of 6-8 animals per experimental group.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: MAXF 449) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 65.00% (Day 80) | Moderate HER2 expression (HER2++; IHC 2+; FISH-) | ||
| Method Description |
All treatments were conducted at day 0 by a single dose, i.v, 3 mg/kg x1 injection into the tail vein, Data, depicted as mean tumour volume, consists of 6-8 animals per experimental group.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: HBCx-34) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 66.70% (Day 31) | Moderate HER2 expression (HER2++; IHC 2+; FISH-) | ||
| Method Description |
All treatments were conducted at day 0 by a single dose, i.v, 3 mg/kg x1 injection into the tail vein, Data, depicted as mean tumour volume, consists of 6-8 animals per experimental group.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: ST313) | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 75.00% (Day 28) | Low HER2 expression (HER2+; IHC +; FISH-) | ||
| Method Description |
All treatments were conducted at day 0 by a single dose, i.v, 10 mg/kg x1 injection into the tail vein, Data, depicted as mean tumour volume, consists of 6-8 animals per experimental group.
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: GXA3057) | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.00% (Day 46) | Moderate HER2 expression (HER2++; IHC 2+; FISH-) | ||
| Method Description |
All treatments were conducted at day 0 by a single dose, i.v, 10 mg/kg x1 injection into the tail vein, Data, depicted as mean tumour volume, consists of 6-8 animals per experimental group.
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: GXA3038) | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 20) | High HER2 expression (HER2+++; IHC 3+; FISH+) | ||
| Method Description |
All treatments were conducted at day 0 by a single dose, i.v, 10 mg/kg x1 injection into the tail vein, Data, depicted as mean tumour volume, consists of 6-8 animals per experimental group.
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: GXA3054) | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 21) | Moderate HER2 expression (HER2++; IHC 2+; FISH+) | ||
| Method Description |
All treatments were conducted at day 0 by a single dose, i.v, 10 mg/kg x1 injection into the tail vein, Data, depicted as mean tumour volume, consists of 6-8 animals per experimental group.
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: GXA3067) | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 41) | High HER2 expression (HER2+++; IHC 3+; FISH+) | ||
| Method Description |
All treatments were conducted at day 0 by a single dose, i.v, 10 mg/kg x1 injection into the tail vein, Data, depicted as mean tumour volume, consists of 6-8 animals per experimental group.
|
||||
| In Vivo Model | Bladder cancer PDX model (PDX: BXF439) | ||||
| Experiment 10 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 32) | Low HER2 expression (HER2+; IHC +; FISH-) | ||
| Method Description |
All treatments were conducted at day 0 by a single dose, i.v, 3 mg/kg x1 injection into the tail vein, Data, depicted as mean tumour volume, consists of 6-8 animals per experimental group.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: MAXF-MX1) | ||||
| Experiment 11 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 40) | Low HER2 expression (HER2+; IHC +; FISH-) | ||
| Method Description |
All treatments were conducted at day 0 by a single dose, i.v, 3 mg/kg x1 injection into the tail vein, Data, depicted as mean tumour volume, consists of 6-8 animals per experimental group.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: HBCx-10) | ||||
Vobramitamab duocarmazine [Phase 2]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05551117 | Phase Status | Phase 2 | ||
| Clinical Description |
A phase 2, randomized, open-label, study of two dose levels of obramitamab duocarmazine in participants with metastatic castration-resistant prostate cancer.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [11] | ||||
| Patients Enrolled |
Patients of multiple tumor types, which included 3 melanoma patients refractory to 2 prior lines of checkpoint therapy.
|
||||
| Administration Dosage |
6 dose cohorts (0.50-4.00 mg/kg) every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03729596 | Phase Status | Phase 1/2 | ||
| Clinical Description |
A phase 1/2, first-in-human, open-label, dose-escalation study of MGC018 (anti-B7-H3 antibody drug conjugate) alone and in combination with MGA012 (anti-PD-1 antibody) in patients with advanced solid tumors.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [12] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05293496 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1/1b dose escalation and cohort expansion study of MGC018 in combination with checkpoint inhibitor in participants with advanced solid tumors.
|
||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.10% (Day 21) | High MET expression (MET+++; IHC H-score=265) | ||
| Method Description |
Female Athymic Nude-Foxn1nu from Envigo (head and neck,triple-negative breast cancer models),68 weeks of age,were used for the PDX studies. Low passage tumor fragments were implanted into stock animals. When tumors reached 1.01.5 cm3,they were reimplanted into prestudy animals unilaterally on the left flank. When tumors reached an average tumor volume of 150-300 mm3,animals were matched by tumor volume into treatment or vehicle control groups. Three animals were assigned to each group and dosed intravenously by tail vein injection (10 mL/kg). Tumor volumes were measured twice weekly by calipers. Prostate cancer subcutaneous PDX model treated with MGC018 or control ADC at 3 mg/kg (QW3).
Click to Show/Hide
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: PDX-PAX-13565) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.60% (Day 42) | Moderate MET expression (MET++; IHC H-score=130) | ||
| Method Description |
Male NOG mice from Taconic (prostate cancer model),68 weeks of age,were used for the PDX studies. Low passage tumor fragments were implanted into stock animals. When tumors reached 1.01.5 cm3,they were reimplanted into prestudy animals unilaterally on the left flank. When tumors reached an average tumor volume of 150-300 mm3,animals were matched by tumor volume into treatment or vehicle control groups. Three animals were assigned to each group and dosed intravenously by tail vein injection (10 mL/kg). Tumor volumes were measured twice weekly by calipers. Head and neck cancer subcutaneous PDX model treated with MGC018 or control ADC at 3 mg/kg (Q2W 2).
Click to Show/Hide
|
||||
| In Vivo Model | Head and neck cancer PDX model | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.20% (Day 10) | High MET expression (MET+++; IHC H-score=240) | ||
| Method Description |
Female Athymic Nude-Foxn1nu from Envigo (head and neck,triple-negative breast cancer models),68 weeks of age,were used for the PDX studies. Low passage tumor fragments were implanted into stock animals. When tumors reached 1.01.5 cm3,they were reimplanted into prestudy animals unilaterally on the left flank. When tumors reached an average tumor volume of 150-300 mm3,animals were matched by tumor volume into treatment or vehicle control groups. Three animals were assigned to each group and dosed intravenously by tail vein injection (10 mL/kg). Tumor volumes were measured twice weekly by calipers. Triple-negative breast cancer subcutaneous PDX model treated with MGC018 or control ADC at 3 mg/kg (QW2).
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 22.40% (Day 50) | High MET expression (MET+++; IHC H-score=287) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). Calu-6 lung cancer subcutaneous xenografts were treated with MGC018 or control ADC at 0.3 mg/kg QWx4.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 30.10% (Day 80) | Moderate MET expression (MET++; IHC H-score=150) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). PA-1 ovarian cancer subcutaneous xenografts were treated with MGC018 or control ADC at 3 mg/kg as a single dose,QW1.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 42.90% (Day 60) | High MET expression (MET+++; IHC H-score=287) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). A-1 ovarian cancer subcutaneous xenografts were treated with MGC018 or control ADC at 3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 43.90% (Day 100) | NegativeCD276 expression (CD276-) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). MDA-MB-468 triple-negative breast cancer orthotopic xenografts were treated with MGC018 or control ADC at 3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 Luc cells | CVCL_0419 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 59.30% (Day 70) | High MET expression (MET+++; IHC H-score=255) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). Calu-6 lung cancer subcutaneous xenografts were treated with MGC018 or control ADC at 3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 62.70% (Day 100) | Moderate MET expression (MET++; IHC H-score=150) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3).MDA-MB-468 triple-negative breast cancer orthotopic xenografts were treated with MGC018 or control ADC at 0.3 mg/kg QW4.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 Luc cells | CVCL_0419 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 62.80% (Day 70) | High MET expression (MET+++; IHC H-score=287) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). PA-1 ovarian cancer subcutaneous xenografts were treated with MGC018 or control ADC at 0.3 mg/kg QW4.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 66.50% (Day 50) | High MET expression (MET+++; IHC H-score=255) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). Calu-6 lung cancer subcutaneous xenografts were treated with MGC018 or control ADC at 3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 71.90% (Day 50) | High MET expression (MET+++; IHC H-score=255) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). Calu-6 lung cancer subcutaneous xenografts were treated with MGC018 or control ADC at 1 mg/kg QWx4.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 76.30% (Day 50) | High MET expression (MET+++; IHC H-score=255) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). Calu-6 lung cancer subcutaneous xenografts were treated with MGC018 or control ADC at 6 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 76.60% (Day 60) | High MET expression (MET+++; IHC H-score=255) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). A375.S2 melanoma subcutaneous xenografts were treated with MGC018 or control ADC at 0.3 mg/kg QW4.
Click to Show/Hide
|
||||
| In Vivo Model | Melanoma CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 82.80% (Day 50) | High MET expression (MET+++; IHC H-score=255) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). Calu-6 lung cancer subcutaneous xenografts were treated with MGC018 or control ADC at 3 mg/kg QWx4.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 83.90% (Day 60) | High MET expression (MET+++; IHC H-score=265) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). A375.S2 melanoma subcutaneous xenografts were treated with MGC018 or control ADC at 1 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Melanoma CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.50% (Day 50) | High MET expression (MET+++; IHC H-score=255) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). Calu-6 lung cancer subcutaneous xenografts were treated with MGC018 or control ADC at 10 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.80% (Day 60) | High MET expression (MET+++; IHC H-score=287) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). A-1 ovarian cancer subcutaneous xenografts were treated with MGC018 or control ADC at 6 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.50% (Day 60) | High MET expression (MET+++; IHC H-score=287) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). A-1 ovarian cancer subcutaneous xenografts were treated with MGC018 or control ADC at 10 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 91.40% (Day 80) | High MET expression (MET+++; IHC H-score=287) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). PA-1 ovarian cancer subcutaneous xenografts were treated with MGC018 or control ADC at 3 mg/kg as a single dose,QW4.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.60% (Day 70) | High MET expression (MET+++; IHC H-score=287) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). PA-1 ovarian cancer subcutaneous xenografts were treated with MGC018 or control ADC at 1 mg/kg QW4.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.70% (Day 70) | High MET expression (MET+++; IHC H-score=255) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). Calu-6 lung cancer subcutaneous xenografts were treated with MGC018 or control ADC at 6 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 20 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.00% (Day 100) | Moderate MET expression (MET++; IHC H-score=150) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3).MDA-MB-468 triple-negative breast cancer orthotopic xenografts were treated with MGC018 or control ADC at 1 mg/kg QW4.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 Luc cells | CVCL_0419 | ||
| Experiment 21 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.60% (Day 80) | High MET expression (MET+++; IHC H-score=287) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). PA-1 ovarian cancer subcutaneous xenografts were treated with MGC018 or control ADC at 3 mg/kg as a single dose,Q2W4.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 22 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.90% (Day 100) | Moderate MET expression (MET++; IHC H-score=150) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). MDA-MB-468 triple-negative breast cancer orthotopic xenografts were treated with MGC018 or control ADC at 6 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 Luc cells | CVCL_0419 | ||
| Experiment 23 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.10% (Day 60) | High MET expression (MET+++; IHC H-score=265) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). A375.S2 melanoma subcutaneous xenografts were treated with MGC018 or control ADC at 3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Melanoma CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 24 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.60% (Day 70) | High MET expression (MET+++; IHC H-score=255) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). Calu-6 lung cancer subcutaneous xenografts were treated with MGC018 or control ADC at 10 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 25 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.70% (Day 60) | High MET expression (MET+++; IHC H-score=265) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). A375.S2 melanoma subcutaneous xenografts were treated with MGC018 or control ADC at 1 mg/kg QW4.
Click to Show/Hide
|
||||
| In Vivo Model | Melanoma CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
181.00 pM
|
High CD276 expression (CD276+++; 138,000 CD276 molecules/cell) | ||
| Method Description |
MGC018-mediated in vitro cytotoxicity was evaluated across a set of tumor cell lines representing multiple cancer types expressing varying levels of B7-H3. MDA-MB-468,A375.S2,PA-1,Calu-6,Hs700T,SW48,and LN-229 tumor cell lines were obtained from ATCC and cultured in DMEM/F-12 media containing 10% FBS. NCI-H1703 and Raji tumor cell lines were obtained from ATCC and cultured in RPMI1640 media containing 10% FBS.
Click to Show/Hide
|
||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
260.00 pM
|
High CD276 expression (CD276+++; 139,000 CD276 molecules/cell) | ||
| Method Description |
MGC018-mediated in vitro cytotoxicity was evaluated across a set of tumor cell lines representing multiple cancer types expressing varying levels of B7-H3. MDA-MB-468,A375.S2,PA-1,Calu-6,Hs700T,SW48,and LN-229 tumor cell lines were obtained from ATCC and cultured in DMEM/F-12 media containing 10% FBS. NCI-H1703 and Raji tumor cell lines were obtained from ATCC and cultured in RPMI1640 media containing 10% FBS.
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
275.00 pM
|
High CD276 expression (CD276+++; 310,000 CD276 molecules/cell) | ||
| Method Description |
MGC018-mediated in vitro cytotoxicity was evaluated across a set of tumor cell lines representing multiple cancer types expressing varying levels of B7-H3. MDA-MB-468,A375.S2,PA-1,Calu-6,Hs700T,SW48,and LN-229 tumor cell lines were obtained from ATCC and cultured in DMEM/F-12 media containing 10% FBS. NCI-H1703 and Raji tumor cell lines were obtained from ATCC and cultured in RPMI1640 media containing 10% FBS.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
319.00 pM
|
High CD276 expression (CD276+++; 122,000 CD276 molecules/cell) | ||
| Method Description |
MGC018-mediated in vitro cytotoxicity was evaluated across a set of tumor cell lines representing multiple cancer types expressing varying levels of B7-H3. MDA-MB-468,A375.S2,PA-1,Calu-6,Hs700T,SW48,and LN-229 tumor cell lines were obtained from ATCC and cultured in DMEM/F-12 media containing 10% FBS. NCI-H1703 and Raji tumor cell lines were obtained from ATCC and cultured in RPMI1640 media containing 10% FBS.
Click to Show/Hide
|
||||
| In Vitro Model | Neoplasm | Hs 700T cells | CVCL_0858 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
585.00 pM
|
Moderate MET expression (MET++; IHC H-score=180) | ||
| Method Description |
MGC018-mediated in vitro cytotoxicity was evaluated across a set of tumor cell lines representing multiple cancer types expressing varying levels of B7-H3. MDA-MB-468,A375.S2,PA-1,Calu-6,Hs700T,SW48,and LN-229 tumor cell lines were obtained from ATCC and cultured in DMEM/F-12 media containing 10% FBS. NCI-H1703 and Raji tumor cell lines were obtained from ATCC and cultured in RPMI1640 media containing 10% FBS.
Click to Show/Hide
|
||||
| In Vitro Model | Lung squamous cell carcinoma | NCI-H1703 cells | CVCL_1490 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
767.00 pM
|
Moderate CD276 expression (CD276++; 57,000 CD276 molecules/cell) | ||
| Method Description |
MGC018-mediated in vitro cytotoxicity was evaluated across a set of tumor cell lines representing multiple cancer types expressing varying levels of B7-H3. MDA-MB-468,A375.S2,PA-1,Calu-6,Hs700T,SW48,and LN-229 tumor cell lines were obtained from ATCC and cultured in DMEM/F-12 media containing 10% FBS. NCI-H1703 and Raji tumor cell lines were obtained from ATCC and cultured in RPMI1640 media containing 10% FBS.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
910.00 pM
|
Moderate CD276 expression (CD276++; 73,700 CD276 molecules/cell) | ||
| Method Description |
MGC018-mediated in vitro cytotoxicity was evaluated across a set of tumor cell lines representing multiple cancer types expressing varying levels of B7-H3. MDA-MB-468,A375.S2,PA-1,Calu-6,Hs700T,SW48,and LN-229 tumor cell lines were obtained from ATCC and cultured in DMEM/F-12 media containing 10% FBS. NCI-H1703 and Raji tumor cell lines were obtained from ATCC and cultured in RPMI1640 media containing 10% FBS.
Click to Show/Hide
|
||||
| In Vitro Model | Glioblastoma | LN-229 cells | CVCL_0393 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1447.00 pM
|
High CD276 expression (CD276+++; 153,000 CD276 molecules/cell) | ||
| Method Description |
MGC018-mediated in vitro cytotoxicity was evaluated across a set of tumor cell lines representing multiple cancer types expressing varying levels of B7-H3. MDA-MB-468,A375.S2,PA-1,Calu-6,Hs700T,SW48,and LN-229 tumor cell lines were obtained from ATCC and cultured in DMEM/F-12 media containing 10% FBS. NCI-H1703 and Raji tumor cell lines were obtained from ATCC and cultured in RPMI1640 media containing 10% FBS.
Click to Show/Hide
|
||||
| In Vitro Model | Colon adenocarcinoma | SW48 cells | CVCL_1724 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 nM | Moderate CD276 expression (CD276++; 59,800 CD276 molecules/cell) | ||
| Method Description |
MGC018-mediated in vitro cytotoxicity was evaluated across a set of tumor cell lines representing multiple cancer types expressing varying levels of B7-H3. MDA-MB-468,A375.S2,PA-1,Calu-6,Hs700T,SW48,and LN-229 tumor cell lines were obtained from ATCC and cultured in DMEM/F-12 media containing 10% FBS. NCI-H1703 and Raji tumor cell lines were obtained from ATCC and cultured in RPMI1640 media containing 10% FBS.
Click to Show/Hide
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
BYON-3521 [Phase 1]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [14] | ||||
| Patients Enrolled |
Patients with previously treated progressive locally advanced or metastatic solid tumors, MET positive.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT05323045 | Phase Status | Phase 1 | ||
| Clinical Description |
A first-in-human dose-escalation and expansion trial with the antibody-drug conjugate BYON3521 to evaluate the safety, pharmacokinetics and efficacy in patients with c-met expressing locally advanced or metastatic solid tumours.
|
||||
SYD-1875 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [15] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04202705 | Phase Status | Phase 1 | ||
| Clinical Description |
A first-in-human dose-escalation and expansion study with the antibody-drug conjugate SYD1875 to evaluate the safety, pharmacokinetics and efficacy in patients with 5T4-expressing locally advanced or metastatic solid tumours.
|
||||
SYD1035 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 28.40% (Day 69) | Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
Tumours were induced subcutaneously by injecting of 10,000,000 LnCap C4.2 cells in 200 uL of RPMI 1640 containing matrigel into the right flank of male CB17.SCID mice. Treatments were started when the tumors reached a mean volume of 100-200 mm3. Mice were randomized according to their individual tumor volume into groups and received a single i.v, 2 mg/kg inection of anti-PSMA ADC.
Click to Show/Hide
|
||||
| In Vivo Model | LNCaP C4-2 CDX model | ||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 28.96% (Day 69) | Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
Tumours were induced subcutaneously by injecting of 10,000,000 LnCap C4.2 cells in 200 uL of RPMI 1640 containing matrigel into the right flank of male CB17.SCID mice. Treatments were started when the tumors reached a mean volume of 100-200 mm3. Mice were randomized according to their individual tumor volume into groups and received a single i.v, 10 mg/kg inection of anti-PSMA ADC.
Click to Show/Hide
|
||||
| In Vivo Model | LNCaP C4-2 CDX model | ||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
82.00%
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
96.00%
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.14 nM
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.38 nM
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 70.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
DU-145 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
SYD998 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 35.67% (Day 69) | Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
Tumours were induced subcutaneously by injecting of 10,000,000 LnCap C4.2 cells in 200 uL of RPMI 1640 containing matrigel into the right flank of male CB17.SCID mice. Treatments were started when the tumors reached a mean volume of 100-200 mm3. Mice were randomized according to their individual tumor volume into groups and received a single i.v, 2 mg/kg inection of anti-PSMA ADC.
Click to Show/Hide
|
||||
| In Vivo Model | LNCaP C4-2 CDX model | ||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.68% (Day 69) | Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
Tumours were induced subcutaneously by injecting of 10,000,000 LnCap C4.2 cells in 200 uL of RPMI 1640 containing matrigel into the right flank of male CB17.SCID mice. Treatments were started when the tumors reached a mean volume of 100-200 mm3. Mice were randomized according to their individual tumor volume into groups and received a single i.v, 10 mg/kg inection of anti-PSMA ADC.
Click to Show/Hide
|
||||
| In Vivo Model | LNCaP C4-2 CDX model | ||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
82.00%
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
97.00%
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.23 nM
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.70 nM
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 70.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
DU-145 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
hmAb-C-DUBA [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 42.39% (Day 59) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
PA-1 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (3 mg/kg) at Day 20.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 43.85% (Day 70) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
PA-1 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (3 mg/kg) at Day 20.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 49.48% (Day 110) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
MDA-MB-468 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (3 mg/kg) at Day 20.
|
||||
| In Vivo Model | MDA-MB-468 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 59.20% (Day 54) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
Calu-6 non-small cell lung carcinoma cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (3 mg/kg) at Day 20.
|
||||
| In Vivo Model | Calu-6 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 76.07% (Day 70) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
PA-1 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (10 mg/kg) at Day 20.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 83.49% (Day 70) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
PA-1 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (10 mg/kg x 2) at Day 20.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 84.42% (Day 62) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
Calu-6 non-small cell lung carcinoma cells were subcutaneously implanted intogroups of mice (n=5) essentially, which then received doses of hmAb-C-DUBA (1 mg/kg x 3) at Day 24, 31, 38 and 45 post inoculation, and the animals were evaluated for tumor volume for up to 62 days.
|
||||
| In Vivo Model | Calu-6 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 85.17% (Day 61) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
A375.52 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (3 mg/kg) at Day 20.
|
||||
| In Vivo Model | A375.52 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.65% (Day 59) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
PA-1 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (10 mg/kg) at Day 20.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.47% (Day 62) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
Calu-6 non-small cell lung carcinoma cells were subcutaneously implanted intogroups of mice (n=5) essentially, which then received doses of hmAb-C-DUBA (3 mg/kg x 3) at Day 24, 31, 38 and 45 post inoculation, and the animals were evaluated for tumor volume for up to 62 days.
|
||||
| In Vivo Model | Calu-6 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 91.81% (Day 54) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
Calu-6 non-small cell lung carcinoma cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (10 mg/kg) at Day 20.
|
||||
| In Vivo Model | Calu-6 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 92.95% (Day 59) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
PA-1 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (6 mg/kg) at Day 20.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.20% (Day 62) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
Calu-6 non-small cell lung carcinoma cells were subcutaneously implanted intogroups of mice (n=5) essentially, which then received doses of hmAb-C-DUBA (6 mg/kg x 3) at Day 24, 31, 38 and 45 post inoculation, and the animals were evaluated for tumor volume for up to 62 days.
|
||||
| In Vivo Model | Calu-6 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.30% (Day 100) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
MDA-MB-468 cells were subcutaneously implanted intogroups of mice (n=5) essentially, which then received doses of hmAb-C-DUBA (3 mg/kg x 3), and the animals were evaluated for tumor volume for up to 110 days.
|
||||
| In Vivo Model | MDA-MB-468 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.92% (Day 61) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
A375.52 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (6 mg/kg) at Day 20.
|
||||
| In Vivo Model | A375.52 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.97% (Day 110) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
MDA-MB-468 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (6 mg/kg) at Day 20.
|
||||
| In Vivo Model | MDA-MB-468 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [17] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.24% (Day 70) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
PA-1 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (10 mg/kg x 4) at Day 20.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
WO2015177360A1 ADC-LC41 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 43.85% (Day 47) | Positive 5T4 expression (5T4+++/++) | ||
| Method Description |
Tumours were induced subcutaneously by injecting of 10,000,000 PA-1 cells in 100 uL of RPMI 1640 containing matrigel into the right flank of male Balb/c nude mice. Treatments were started when the tumors reached a mean volume of 200-300 mm3. Mice were randomized according to their individual tumor volume into groups and received a single i.v, 3 mg/kg inection of anti-5T4 ADC.
Click to Show/Hide
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 85.04% (Day 47) | Positive 5T4 expression (5T4+++/++) | ||
| Method Description |
Tumours were induced subcutaneously by injecting of 10,000,000 PA-1 cells in 100 uL of RPMI 1640 containing matrigel into the right flank of male Balb/c nude mice. Treatments were started when the tumors reached a mean volume of 200-300 mm3. Mice were randomized according to their individual tumor volume into groups and received a single i.v, 10 mg/kg inection of anti-5T4 ADC.
Click to Show/Hide
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
50.00%
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
80.00%
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
91.00%
|
Positive 5T4 expression (5T4+++/++) | ||
| Method Description |
MDA-MB-468 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
98.00%
|
Negative 5T4 expression (5T4-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, 5T4-negative SK-MEL-30 cells (2,000 cells/well) was cultured with the ADCs for 6 days, and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Cutaneous melanoma | SK-MEL-30 cells | CVCL_0039 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.09 nM
|
Positive 5T4 expression (5T4+++/++) | ||
| Method Description |
MDA-MB-468 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.31 nM
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.35 nM
|
Negative 5T4 expression (5T4-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, 5T4-negative SK-MEL-30 cells (2,000 cells/well) was cultured with the ADCs for 6 days, and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Cutaneous melanoma | SK-MEL-30 cells | CVCL_0039 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 70.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
DU-145 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 90.00 nM | Negative 5T4 expression (5T4-) | ||
| Method Description |
SK-MEL-30 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Cutaneous melanoma | SK-MEL-30 cells | CVCL_0039 | ||
SYD1091 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 67.53% (Day 69) | Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
Tumours were induced subcutaneously by injecting of 10,000,000 LnCap C4.2 cells in 200 uL of RPMI 1640 containing matrigel into the right flank of male CB17.SCID mice. Treatments were started when the tumors reached a mean volume of 100-200 mm3. Mice were randomized according to their individual tumor volume into groups and received a single i.v, 2 mg/kg inection of anti-PSMA ADC.
Click to Show/Hide
|
||||
| In Vivo Model | LNCaP C4-2 CDX model | ||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.44% (Day 69) | Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
Tumours were induced subcutaneously by injecting of 10,000,000 LnCap C4.2 cells in 200 uL of RPMI 1640 containing matrigel into the right flank of male CB17.SCID mice. Treatments were started when the tumors reached a mean volume of 100-200 mm3. Mice were randomized according to their individual tumor volume into groups and received a single i.v, 10 mg/kg inection of anti-PSMA ADC.
Click to Show/Hide
|
||||
| In Vivo Model | LNCaP C4-2 CDX model | ||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
59.00%
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
78.00%
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.25 nM
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.00 nM
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 70.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
DU-145 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
WO2015177360A1 ADC-H8-HC41 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 72.01% (Day 47) | Positive 5T4 expression (5T4+++/++) | ||
| Method Description |
Tumours were induced subcutaneously by injecting of 10,000,000 PA-1 cells in 100 uL of RPMI 1640 containing matrigel into the right flank of male Balb/c nude mice. Treatments were started when the tumors reached a mean volume of 200-300 mm3. Mice were randomized according to their individual tumor volume into groups and received a single i.v, 3 mg/kg inection of anti-5T4 ADC.
Click to Show/Hide
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 86.18% (Day 47) | Positive 5T4 expression (5T4+++/++) | ||
| Method Description |
Tumours were induced subcutaneously by injecting of 10,000,000 PA-1 cells in 100 uL of RPMI 1640 containing matrigel into the right flank of male Balb/c nude mice. Treatments were started when the tumors reached a mean volume of 200-300 mm3. Mice were randomized according to their individual tumor volume into groups and received a single i.v, 10 mg/kg inection of anti-5T4 ADC.
Click to Show/Hide
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
88.00%
|
Positive 5T4 expression (5T4+++/++) | ||
| Method Description |
MDA-MB-468 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
98.00%
|
Negative 5T4 expression (5T4-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, 5T4-negative SK-MEL-30 cells (2,000 cells/well) was cultured with the ADCs for 6 days, and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Cutaneous melanoma | SK-MEL-30 cells | CVCL_0039 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07 nM
|
Positive 5T4 expression (5T4+++/++) | ||
| Method Description |
MDA-MB-468 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.27 nM
|
Negative 5T4 expression (5T4-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, 5T4-negative SK-MEL-30 cells (2,000 cells/well) was cultured with the ADCs for 6 days, and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Cutaneous melanoma | SK-MEL-30 cells | CVCL_0039 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 90.00 nM | Negative 5T4 expression (5T4-) | ||
| Method Description |
SK-MEL-30 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Cutaneous melanoma | SK-MEL-30 cells | CVCL_0039 | ||
WO2015177360A1 ADC-HC375 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
45.00%
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
81.00%
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.25 nM
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 70.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
DU-145 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
WO2015177360A1 ADC-HC152 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
50.00%
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
78.00%
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.44 nM
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 70.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
DU-145 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
WO2015177360A1 ADC-LC40 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
50.00%
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
79.00%
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
80.00%
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
82.00%
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
83.00%
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
96.00%
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
97.00%
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
98.00%
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.17 nM
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.24 nM
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.26 nM
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.30 nM
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.48 nM
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.51 nM
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.11 nM
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 70.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
DU-145 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 70.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
DU-145 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 70.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
DU-145 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 20 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 70.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
DU-145 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
WO2015177360A1 ADC-HC236 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
76.00%
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
100.00%
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.22 nM
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.08 nM
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 70.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
DU-145 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
WO2015177360A1 ADC-HC153 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
79.00%
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
98.00%
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.34 nM
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.76 nM
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 70.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
DU-145 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
WO2015177360A1 ADC-HC247 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
82.00%
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.01 nM
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 70.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
DU-145 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
WO2015177360A1 ADC-HC376 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
82.00%
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
98.00%
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.20 nM
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.60 nM
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 70.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
DU-145 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
WO2015177360A1 ADC-HC339 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
83.00%
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
99.00%
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.12 nM
|
Positive PSMA expression (PSMA+++/++) | ||
| Method Description |
LNCaP C4-2 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Lymph node metastasis of prostate carcinoma | LNCaP C4-2 cells | CVCL_4782 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.00 nM
|
Negative PSMA expression (PSMA-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, PSMA-negative DU-145 cells (1,000 cells/well) was cultured with the ADCs for 6 days,and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 70.00 nM | Negative PSMA expression (PSMA-) | ||
| Method Description |
DU-145 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
WO2015177360A1 ADC-H8-HC40 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
88.00%
|
Positive 5T4 expression (5T4+++/++) | ||
| Method Description |
MDA-MB-468 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Max inhibition rate (MIR) |
93.00%
|
Negative 5T4 expression (5T4-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, 5T4-negative SK-MEL-30 cells (2,000 cells/well) was cultured with the ADCs for 6 days, and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Cutaneous melanoma | SK-MEL-30 cells | CVCL_0039 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07 nM
|
Positive 5T4 expression (5T4+++/++) | ||
| Method Description |
MDA-MB-468 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.98 nM
|
Negative 5T4 expression (5T4-) | ||
| Method Description |
To assess the sensitivity towards cathepsin B, the ADCs were treated for 2 minutes and 4 hours with activated cathepsin B. To measure release of the respective free toxins DUBA or MMAE, 5T4-negative SK-MEL-30 cells (2,000 cells/well) was cultured with the ADCs for 6 days, and the cell viability was measured after 6 days using the CellTiter-GloTM (CTG) assay kit.
Click to Show/Hide
|
||||
| In Vitro Model | Cutaneous melanoma | SK-MEL-30 cells | CVCL_0039 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [16] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 90.00 nM | Negative 5T4 expression (5T4-) | ||
| Method Description |
SK-MEL-30 cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Cutaneous melanoma | SK-MEL-30 cells | CVCL_0039 | ||
References
