Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0EMQPK
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
SYD-1875
|
|||||
| Synonyms |
Anti-5T4 antibody drug conjugate; SYD 1875; SYD-1875; SYD18 75; SYD1875
Click to Show/Hide
|
|||||
| Organization |
Synthon BV; Byondis BV
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
1.9
|
|||||
| Structure |
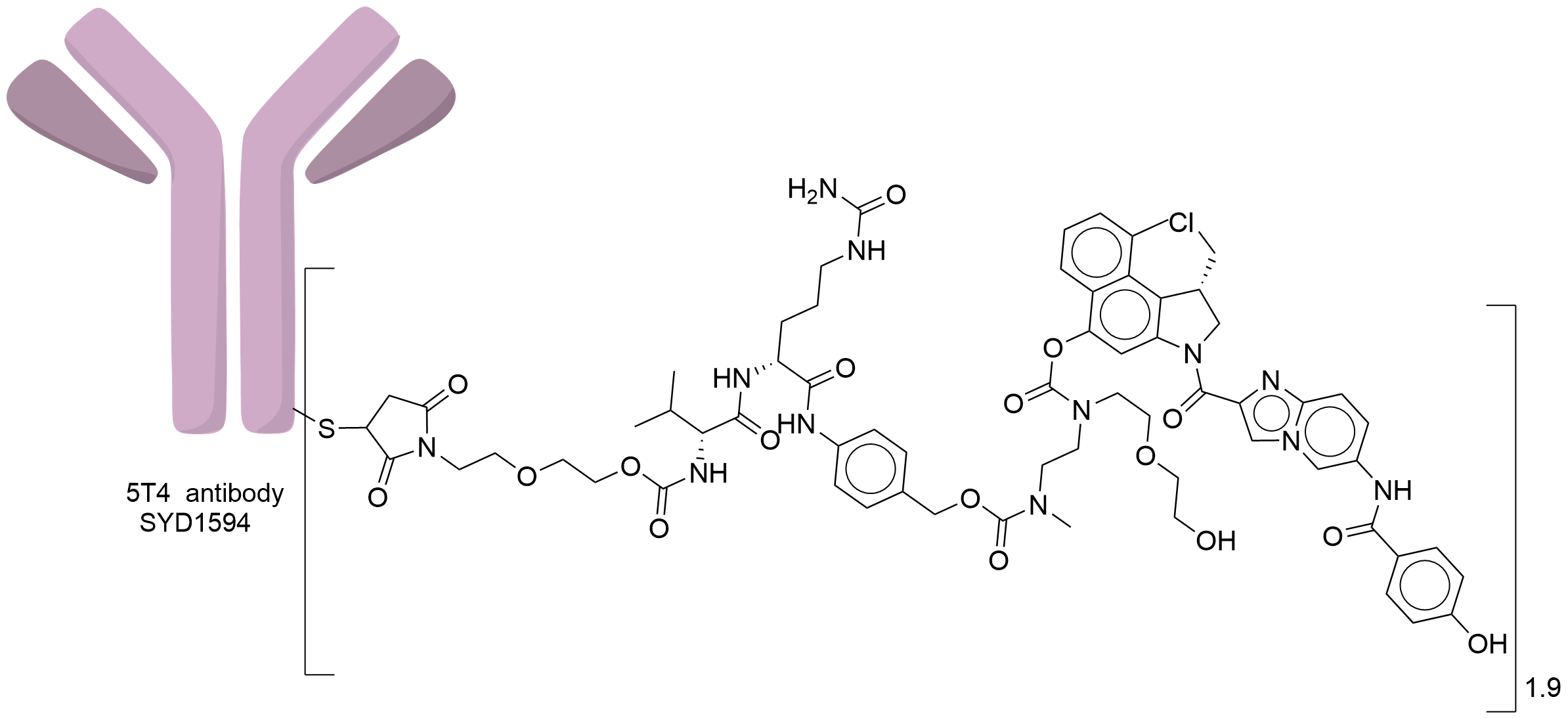
|
|||||
| Antibody Name |
SYD1594
|
Antibody Info | ||||
| Antigen Name |
Trophoblast glycoprotein (TPBG)
|
Antigen Info | ||||
| Payload Name |
seco-DUBA
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
Mal-PEG2-Val-Cit-PABA-Cyclization Spacer
|
Linker Info | ||||
| Conjugate Type |
Reactive Cysteines
|
|||||
| Combination Type |
duocarmazine
|
|||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04202705 | Clinical Status | Phase 1 | ||
| Clinical Description | A first-in-human dose-escalation and expansion study with the antibody-drug conjugate SYD1875 to evaluate the safety, pharmacokinetics and efficacy in patients with 5T4-expressing locally advanced or metastatic solid tumours. | ||||
References
