Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0DEGEG
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Vobramitamab duocarmazine
|
|||||
| Synonyms |
AEX4089DC1; Anti-B7-H3 antibody drug conjugate; MGC 018; MGC-018; MGC018; Vobramitamab duocarmazine
Click to Show/Hide
|
|||||
| Organization |
MacroGenics, Inc.
|
|||||
| Drug Status |
Phase 2
|
|||||
| Indication |
In total 8 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2.7
|
|||||
| Structure |
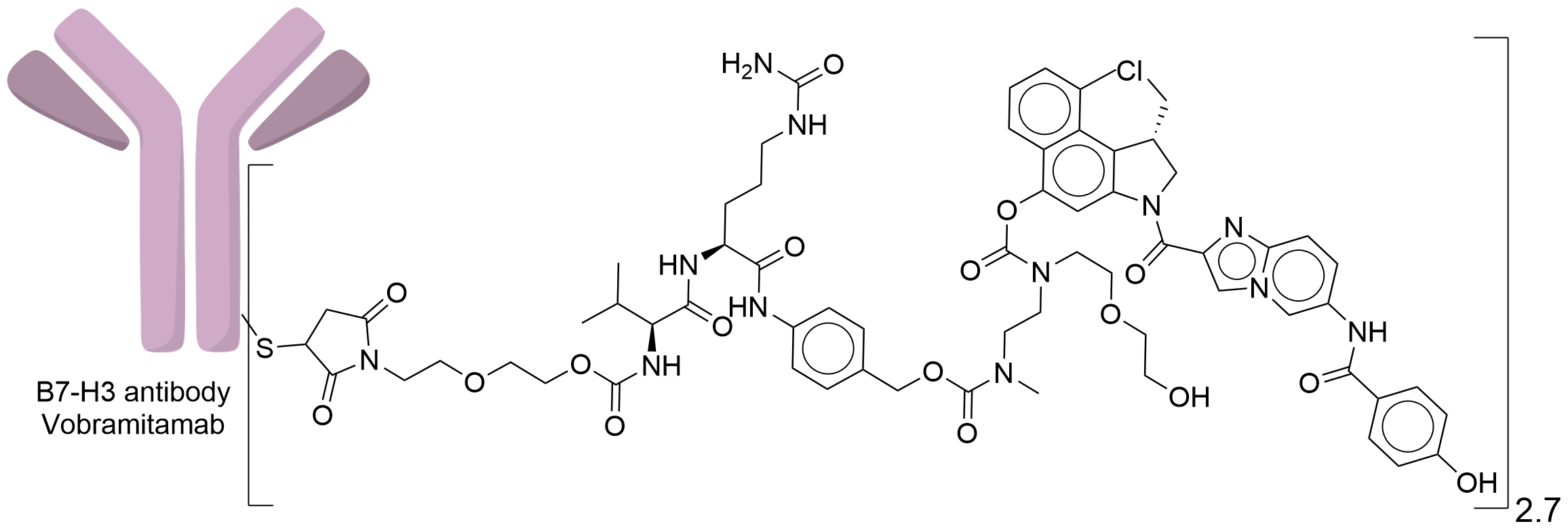
|
|||||
| Antibody Name |
Vobramitamab
|
Antibody Info | ||||
| Antigen Name |
CD276 antigen (CD276)
|
Antigen Info | ||||
| Payload Name |
seco-DUBA
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
Mal-PEG2-Val-Cit-PABA-Cyclization Spacer
|
Linker Info | ||||
| Conjugate Type |
Random Lysines
|
|||||
| Combination Type |
duocarmazine
|
|||||
| Puchem SID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Patient-derived Xenograft Model
Discovered Using Cell Line-derived Xenograft Model
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05551117 | Clinical Status | Phase 2 | ||
| Clinical Description | A phase 2, randomized, open-label, study of two dose levels of obramitamab duocarmazine in participants with metastatic castration-resistant prostate cancer. | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Patients Enrolled |
Patients of multiple tumor types, which included 3 melanoma patients refractory to 2 prior lines of checkpoint therapy.
|
||||
| Administration Dosage |
6 dose cohorts (0.50-4.00 mg/kg) every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03729596 | Clinical Status | Phase 1/2 | ||
| Clinical Description | A phase 1/2, first-in-human, open-label, dose-escalation study of MGC018 (anti-B7-H3 antibody drug conjugate) alone and in combination with MGA012 (anti-PD-1 antibody) in patients with advanced solid tumors. | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05293496 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/1b dose escalation and cohort expansion study of MGC018 in combination with checkpoint inhibitor in participants with advanced solid tumors. | ||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.10% (Day 21) | High MET expression (MET+++; IHC H-score=265) | ||
| Method Description |
Female Athymic Nude-Foxn1nu from Envigo (head and neck,triple-negative breast cancer models),68 weeks of age,were used for the PDX studies. Low passage tumor fragments were implanted into stock animals. When tumors reached 1.01.5 cm3,they were reimplanted into prestudy animals unilaterally on the left flank. When tumors reached an average tumor volume of 150-300 mm3,animals were matched by tumor volume into treatment or vehicle control groups. Three animals were assigned to each group and dosed intravenously by tail vein injection (10 mL/kg). Tumor volumes were measured twice weekly by calipers. Prostate cancer subcutaneous PDX model treated with MGC018 or control ADC at 3 mg/kg (QW3).
Click to Show/Hide
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: PDX-PAX-13565) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.60% (Day 42) | Moderate MET expression (MET++; IHC H-score=130) | ||
| Method Description |
Male NOG mice from Taconic (prostate cancer model),68 weeks of age,were used for the PDX studies. Low passage tumor fragments were implanted into stock animals. When tumors reached 1.01.5 cm3,they were reimplanted into prestudy animals unilaterally on the left flank. When tumors reached an average tumor volume of 150-300 mm3,animals were matched by tumor volume into treatment or vehicle control groups. Three animals were assigned to each group and dosed intravenously by tail vein injection (10 mL/kg). Tumor volumes were measured twice weekly by calipers. Head and neck cancer subcutaneous PDX model treated with MGC018 or control ADC at 3 mg/kg (Q2W 2).
Click to Show/Hide
|
||||
| In Vivo Model | Head and neck cancer PDX model | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.20% (Day 10) | High MET expression (MET+++; IHC H-score=240) | ||
| Method Description |
Female Athymic Nude-Foxn1nu from Envigo (head and neck,triple-negative breast cancer models),68 weeks of age,were used for the PDX studies. Low passage tumor fragments were implanted into stock animals. When tumors reached 1.01.5 cm3,they were reimplanted into prestudy animals unilaterally on the left flank. When tumors reached an average tumor volume of 150-300 mm3,animals were matched by tumor volume into treatment or vehicle control groups. Three animals were assigned to each group and dosed intravenously by tail vein injection (10 mL/kg). Tumor volumes were measured twice weekly by calipers. Triple-negative breast cancer subcutaneous PDX model treated with MGC018 or control ADC at 3 mg/kg (QW2).
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 22.40% (Day 50) | High MET expression (MET+++; IHC H-score=287) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). Calu-6 lung cancer subcutaneous xenografts were treated with MGC018 or control ADC at 0.3 mg/kg QWx4.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 30.10% (Day 80) | Moderate MET expression (MET++; IHC H-score=150) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). PA-1 ovarian cancer subcutaneous xenografts were treated with MGC018 or control ADC at 3 mg/kg as a single dose,QW1.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 42.90% (Day 60) | High MET expression (MET+++; IHC H-score=287) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). A-1 ovarian cancer subcutaneous xenografts were treated with MGC018 or control ADC at 3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 43.90% (Day 100) | NegativeCD276 expression (CD276-) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). MDA-MB-468 triple-negative breast cancer orthotopic xenografts were treated with MGC018 or control ADC at 3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 Luc cells | CVCL_0419 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 59.30% (Day 70) | High MET expression (MET+++; IHC H-score=255) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). Calu-6 lung cancer subcutaneous xenografts were treated with MGC018 or control ADC at 3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 62.70% (Day 100) | Moderate MET expression (MET++; IHC H-score=150) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3).MDA-MB-468 triple-negative breast cancer orthotopic xenografts were treated with MGC018 or control ADC at 0.3 mg/kg QW4.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 Luc cells | CVCL_0419 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 62.80% (Day 70) | High MET expression (MET+++; IHC H-score=287) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). PA-1 ovarian cancer subcutaneous xenografts were treated with MGC018 or control ADC at 0.3 mg/kg QW4.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 66.50% (Day 50) | High MET expression (MET+++; IHC H-score=255) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). Calu-6 lung cancer subcutaneous xenografts were treated with MGC018 or control ADC at 3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 71.90% (Day 50) | High MET expression (MET+++; IHC H-score=255) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). Calu-6 lung cancer subcutaneous xenografts were treated with MGC018 or control ADC at 1 mg/kg QWx4.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 76.30% (Day 50) | High MET expression (MET+++; IHC H-score=255) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). Calu-6 lung cancer subcutaneous xenografts were treated with MGC018 or control ADC at 6 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 76.60% (Day 60) | High MET expression (MET+++; IHC H-score=255) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). A375.S2 melanoma subcutaneous xenografts were treated with MGC018 or control ADC at 0.3 mg/kg QW4.
Click to Show/Hide
|
||||
| In Vivo Model | Melanoma CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 82.80% (Day 50) | High MET expression (MET+++; IHC H-score=255) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). Calu-6 lung cancer subcutaneous xenografts were treated with MGC018 or control ADC at 3 mg/kg QWx4.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 83.90% (Day 60) | High MET expression (MET+++; IHC H-score=265) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). A375.S2 melanoma subcutaneous xenografts were treated with MGC018 or control ADC at 1 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Melanoma CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.50% (Day 50) | High MET expression (MET+++; IHC H-score=255) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). Calu-6 lung cancer subcutaneous xenografts were treated with MGC018 or control ADC at 10 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.80% (Day 60) | High MET expression (MET+++; IHC H-score=287) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). A-1 ovarian cancer subcutaneous xenografts were treated with MGC018 or control ADC at 6 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.50% (Day 60) | High MET expression (MET+++; IHC H-score=287) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). A-1 ovarian cancer subcutaneous xenografts were treated with MGC018 or control ADC at 10 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 91.40% (Day 80) | High MET expression (MET+++; IHC H-score=287) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). PA-1 ovarian cancer subcutaneous xenografts were treated with MGC018 or control ADC at 3 mg/kg as a single dose,QW4.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.60% (Day 70) | High MET expression (MET+++; IHC H-score=287) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). PA-1 ovarian cancer subcutaneous xenografts were treated with MGC018 or control ADC at 1 mg/kg QW4.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.70% (Day 70) | High MET expression (MET+++; IHC H-score=255) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). Calu-6 lung cancer subcutaneous xenografts were treated with MGC018 or control ADC at 6 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 20 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.00% (Day 100) | Moderate MET expression (MET++; IHC H-score=150) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3).MDA-MB-468 triple-negative breast cancer orthotopic xenografts were treated with MGC018 or control ADC at 1 mg/kg QW4.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 Luc cells | CVCL_0419 | ||
| Experiment 21 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.60% (Day 80) | High MET expression (MET+++; IHC H-score=287) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). PA-1 ovarian cancer subcutaneous xenografts were treated with MGC018 or control ADC at 3 mg/kg as a single dose,Q2W4.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 22 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.90% (Day 100) | Moderate MET expression (MET++; IHC H-score=150) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). MDA-MB-468 triple-negative breast cancer orthotopic xenografts were treated with MGC018 or control ADC at 6 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 Luc cells | CVCL_0419 | ||
| Experiment 23 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.10% (Day 60) | High MET expression (MET+++; IHC H-score=265) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). A375.S2 melanoma subcutaneous xenografts were treated with MGC018 or control ADC at 3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Melanoma CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 24 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.60% (Day 70) | High MET expression (MET+++; IHC H-score=255) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). Calu-6 lung cancer subcutaneous xenografts were treated with MGC018 or control ADC at 10 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 25 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.70% (Day 60) | High MET expression (MET+++; IHC H-score=265) | ||
| Method Description |
Human tumor cells (5x106) were resuspended in 1:1 medium (DMEM/F-12) and Matrigel basement membrane matrix (Corning) and implanted subcutaneously into the flank (A375.S2,Calu-6,PA-1) or mammary fat pad (MDA-MB-468) of mice. Mice were randomized into groups of 5-7 individuals per group. ADCs or vehicle control (PBS) were administered intravenously by tail vein injection (10 mL/kg) following growth of established tumors (100-150 mm3). A375.S2 melanoma subcutaneous xenografts were treated with MGC018 or control ADC at 1 mg/kg QW4.
Click to Show/Hide
|
||||
| In Vivo Model | Melanoma CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 181.00 pM | High CD276 expression (CD276+++; 138,000 CD276 molecules/cell) | ||
| Method Description |
MGC018-mediated in vitro cytotoxicity was evaluated across a set of tumor cell lines representing multiple cancer types expressing varying levels of B7-H3. MDA-MB-468,A375.S2,PA-1,Calu-6,Hs700T,SW48,and LN-229 tumor cell lines were obtained from ATCC and cultured in DMEM/F-12 media containing 10% FBS. NCI-H1703 and Raji tumor cell lines were obtained from ATCC and cultured in RPMI1640 media containing 10% FBS.
Click to Show/Hide
|
||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 260.00 pM | High CD276 expression (CD276+++; 139,000 CD276 molecules/cell) | ||
| Method Description |
MGC018-mediated in vitro cytotoxicity was evaluated across a set of tumor cell lines representing multiple cancer types expressing varying levels of B7-H3. MDA-MB-468,A375.S2,PA-1,Calu-6,Hs700T,SW48,and LN-229 tumor cell lines were obtained from ATCC and cultured in DMEM/F-12 media containing 10% FBS. NCI-H1703 and Raji tumor cell lines were obtained from ATCC and cultured in RPMI1640 media containing 10% FBS.
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 275.00 pM | High CD276 expression (CD276+++; 310,000 CD276 molecules/cell) | ||
| Method Description |
MGC018-mediated in vitro cytotoxicity was evaluated across a set of tumor cell lines representing multiple cancer types expressing varying levels of B7-H3. MDA-MB-468,A375.S2,PA-1,Calu-6,Hs700T,SW48,and LN-229 tumor cell lines were obtained from ATCC and cultured in DMEM/F-12 media containing 10% FBS. NCI-H1703 and Raji tumor cell lines were obtained from ATCC and cultured in RPMI1640 media containing 10% FBS.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 319.00 pM | High CD276 expression (CD276+++; 122,000 CD276 molecules/cell) | ||
| Method Description |
MGC018-mediated in vitro cytotoxicity was evaluated across a set of tumor cell lines representing multiple cancer types expressing varying levels of B7-H3. MDA-MB-468,A375.S2,PA-1,Calu-6,Hs700T,SW48,and LN-229 tumor cell lines were obtained from ATCC and cultured in DMEM/F-12 media containing 10% FBS. NCI-H1703 and Raji tumor cell lines were obtained from ATCC and cultured in RPMI1640 media containing 10% FBS.
Click to Show/Hide
|
||||
| In Vitro Model | Neoplasm | Hs 700T cells | CVCL_0858 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 585.00 pM | Moderate MET expression (MET++; IHC H-score=180) | ||
| Method Description |
MGC018-mediated in vitro cytotoxicity was evaluated across a set of tumor cell lines representing multiple cancer types expressing varying levels of B7-H3. MDA-MB-468,A375.S2,PA-1,Calu-6,Hs700T,SW48,and LN-229 tumor cell lines were obtained from ATCC and cultured in DMEM/F-12 media containing 10% FBS. NCI-H1703 and Raji tumor cell lines were obtained from ATCC and cultured in RPMI1640 media containing 10% FBS.
Click to Show/Hide
|
||||
| In Vitro Model | Lung squamous cell carcinoma | NCI-H1703 cells | CVCL_1490 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 767.00 pM | Moderate CD276 expression (CD276++; 57,000 CD276 molecules/cell) | ||
| Method Description |
MGC018-mediated in vitro cytotoxicity was evaluated across a set of tumor cell lines representing multiple cancer types expressing varying levels of B7-H3. MDA-MB-468,A375.S2,PA-1,Calu-6,Hs700T,SW48,and LN-229 tumor cell lines were obtained from ATCC and cultured in DMEM/F-12 media containing 10% FBS. NCI-H1703 and Raji tumor cell lines were obtained from ATCC and cultured in RPMI1640 media containing 10% FBS.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 910.00 pM | Moderate CD276 expression (CD276++; 73,700 CD276 molecules/cell) | ||
| Method Description |
MGC018-mediated in vitro cytotoxicity was evaluated across a set of tumor cell lines representing multiple cancer types expressing varying levels of B7-H3. MDA-MB-468,A375.S2,PA-1,Calu-6,Hs700T,SW48,and LN-229 tumor cell lines were obtained from ATCC and cultured in DMEM/F-12 media containing 10% FBS. NCI-H1703 and Raji tumor cell lines were obtained from ATCC and cultured in RPMI1640 media containing 10% FBS.
Click to Show/Hide
|
||||
| In Vitro Model | Glioblastoma | LN-229 cells | CVCL_0393 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 1447.00 pM | High CD276 expression (CD276+++; 153,000 CD276 molecules/cell) | ||
| Method Description |
MGC018-mediated in vitro cytotoxicity was evaluated across a set of tumor cell lines representing multiple cancer types expressing varying levels of B7-H3. MDA-MB-468,A375.S2,PA-1,Calu-6,Hs700T,SW48,and LN-229 tumor cell lines were obtained from ATCC and cultured in DMEM/F-12 media containing 10% FBS. NCI-H1703 and Raji tumor cell lines were obtained from ATCC and cultured in RPMI1640 media containing 10% FBS.
Click to Show/Hide
|
||||
| In Vitro Model | Colon adenocarcinoma | SW48 cells | CVCL_1724 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 nM | Moderate CD276 expression (CD276++; 59,800 CD276 molecules/cell) | ||
| Method Description |
MGC018-mediated in vitro cytotoxicity was evaluated across a set of tumor cell lines representing multiple cancer types expressing varying levels of B7-H3. MDA-MB-468,A375.S2,PA-1,Calu-6,Hs700T,SW48,and LN-229 tumor cell lines were obtained from ATCC and cultured in DMEM/F-12 media containing 10% FBS. NCI-H1703 and Raji tumor cell lines were obtained from ATCC and cultured in RPMI1640 media containing 10% FBS.
Click to Show/Hide
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
References
