Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0XTMJF
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
hmAb-C-DUBA
|
|||||
| Synonyms |
hmAb-C DUBA
Click to Show/Hide
|
|||||
| Organization |
Macrogenics, Inc.
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 6 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2-3
|
|||||
| Structure |
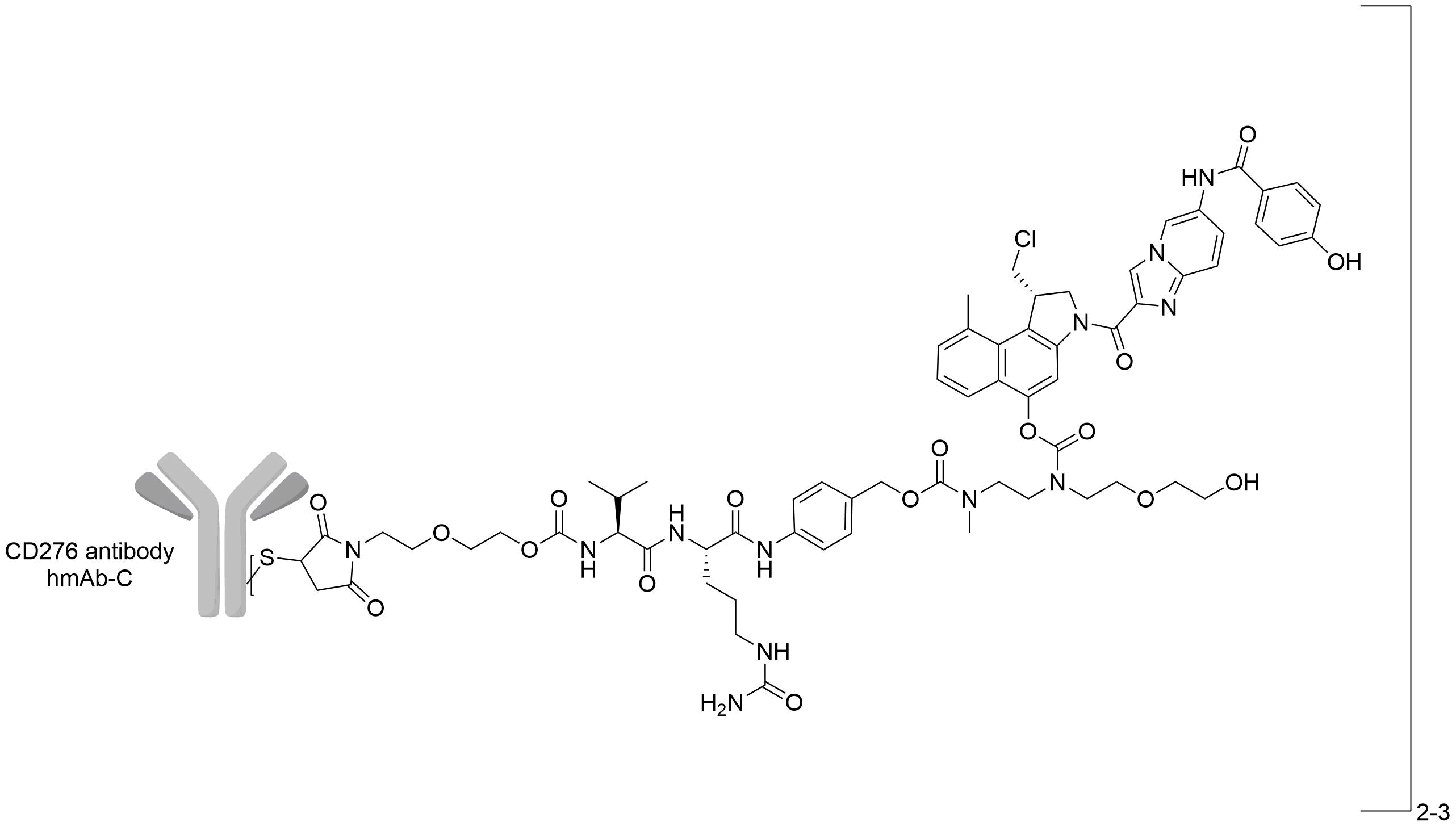
|
|||||
| Antibody Name |
hmAb-C
|
Antibody Info | ||||
| Antigen Name |
CD276 antigen (CD276)
|
Antigen Info | ||||
| Payload Name |
seco-DUBA
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
Mal-PEG2-Val-Cit-PABA-Cyclization Spacer
|
Linker Info | ||||
| Conjugate Type |
Random conjugation through reduced inter-chain cysteines.
|
|||||
| Combination Type |
Duocarmazine
|
|||||
General Information of The Activity Data Related to This ADC
Discovered Using Cell Line-derived Xenograft Model
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 42.39% (Day 59) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
PA-1 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (3 mg/kg) at Day 20.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 43.85% (Day 70) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
PA-1 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (3 mg/kg) at Day 20.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 49.48% (Day 110) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
MDA-MB-468 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (3 mg/kg) at Day 20.
|
||||
| In Vivo Model | MDA-MB-468 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 59.20% (Day 54) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
Calu-6 non-small cell lung carcinoma cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (3 mg/kg) at Day 20.
|
||||
| In Vivo Model | Calu-6 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 76.07% (Day 70) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
PA-1 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (10 mg/kg) at Day 20.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 83.49% (Day 70) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
PA-1 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (10 mg/kg x 2) at Day 20.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 84.42% (Day 62) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
Calu-6 non-small cell lung carcinoma cells were subcutaneously implanted intogroups of mice (n=5) essentially, which then received doses of hmAb-C-DUBA (1 mg/kg x 3) at Day 24, 31, 38 and 45 post inoculation, and the animals were evaluated for tumor volume for up to 62 days.
|
||||
| In Vivo Model | Calu-6 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 85.17% (Day 61) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
A375.52 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (3 mg/kg) at Day 20.
|
||||
| In Vivo Model | A375.52 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.65% (Day 59) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
PA-1 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (10 mg/kg) at Day 20.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.47% (Day 62) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
Calu-6 non-small cell lung carcinoma cells were subcutaneously implanted intogroups of mice (n=5) essentially, which then received doses of hmAb-C-DUBA (3 mg/kg x 3) at Day 24, 31, 38 and 45 post inoculation, and the animals were evaluated for tumor volume for up to 62 days.
|
||||
| In Vivo Model | Calu-6 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 91.81% (Day 54) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
Calu-6 non-small cell lung carcinoma cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (10 mg/kg) at Day 20.
|
||||
| In Vivo Model | Calu-6 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 92.95% (Day 59) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
PA-1 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (6 mg/kg) at Day 20.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.20% (Day 62) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
Calu-6 non-small cell lung carcinoma cells were subcutaneously implanted intogroups of mice (n=5) essentially, which then received doses of hmAb-C-DUBA (6 mg/kg x 3) at Day 24, 31, 38 and 45 post inoculation, and the animals were evaluated for tumor volume for up to 62 days.
|
||||
| In Vivo Model | Calu-6 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.30% (Day 100) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
MDA-MB-468 cells were subcutaneously implanted intogroups of mice (n=5) essentially, which then received doses of hmAb-C-DUBA (3 mg/kg x 3), and the animals were evaluated for tumor volume for up to 110 days.
|
||||
| In Vivo Model | MDA-MB-468 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.92% (Day 61) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
A375.52 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (6 mg/kg) at Day 20.
|
||||
| In Vivo Model | A375.52 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.97% (Day 110) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
MDA-MB-468 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (6 mg/kg) at Day 20.
|
||||
| In Vivo Model | MDA-MB-468 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.24% (Day 70) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
PA-1 cells were subcutaneously implanted into groups of mice(n=7), which then received a single dose of hmAb-C-DUBA or Ctrl-DUBA (10 mg/kg x 4) at Day 20.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
References
