Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0ZUQNL
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
BAT8001
|
|||||
| Synonyms |
Anti-ErbB2 antibody drug conjugate; Anti-HER2 antibody drug conjugate; BAT 8001; BAT-8001; BAT8001
Click to Show/Hide
|
|||||
| Organization |
Bio-Thera Solutions, Ltd.
|
|||||
| Drug Status |
Phase 3 (Terminated)
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3.5
|
|||||
| Structure |
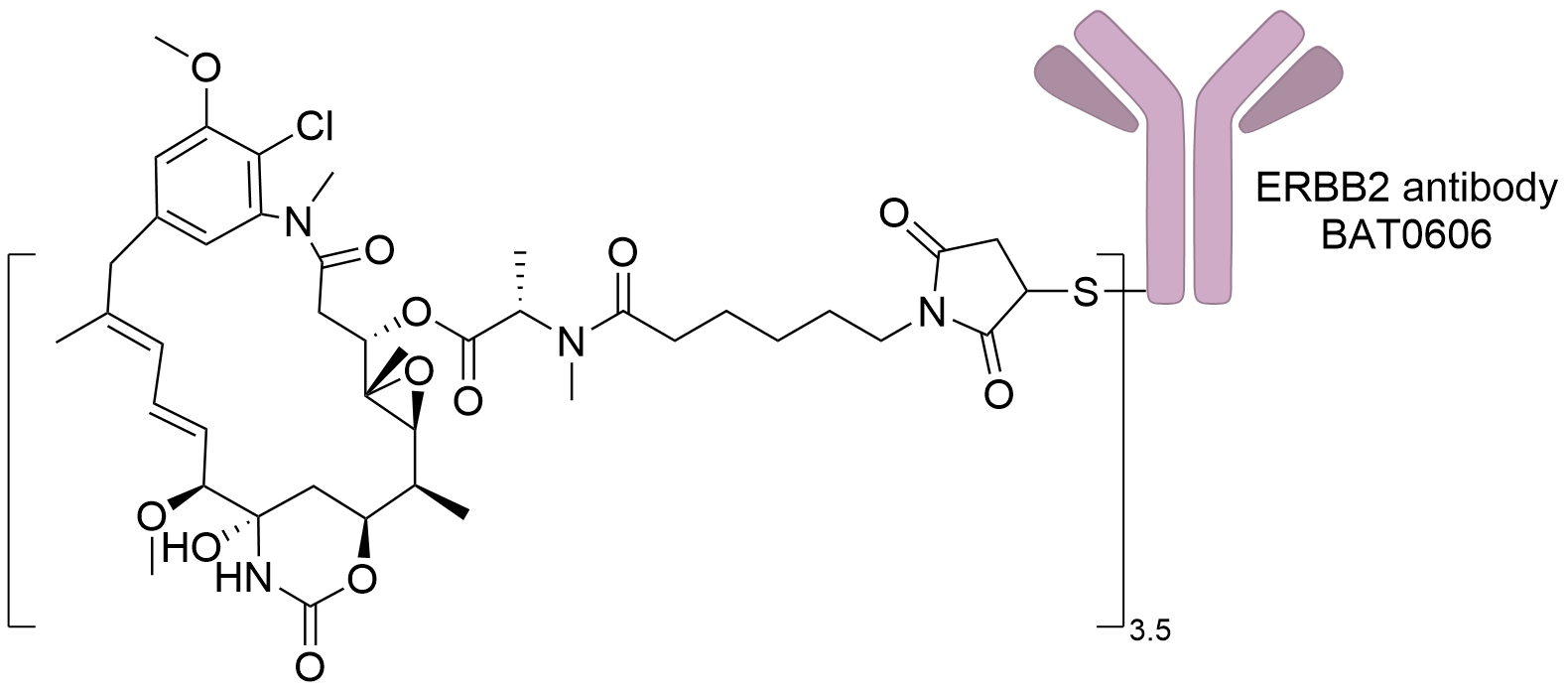
|
|||||
| Antibody Name |
BAT0606
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
Maytansinoid derivative of batansine
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
6-Maleimidocaproic acid
|
Linker Info | ||||
| Conjugate Type |
Random Lysines
|
|||||
| Combination Type |
batansine
|
|||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
41.40%
|
|||
| Patients Enrolled |
HER2-positive locally advanced or metastatic breast cancer.
|
||||
| Administration Dosage |
Patients received BAT8001 intravenously in a 21-day cycle, with dose escalation in 5 cohorts: 1.20, 2.40, 3.60, 4.80, and 6.00 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT04189211 | Clinical Status | Phase 1 | ||
| Clinical Description | An open-label, dose escalation phase 1 clinical trial on safety, tolerability and pharmacokinetics of BAT8001 for injection in patients with HER2-positive solid tumors. | ||||
| Primary Endpoint |
For BAT8001, 3.60 mg/kg was determined to be the MTD.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04185649 | Clinical Status | Phase 3 | ||
| Clinical Description | A clinical study evaluating the efficacy and safety of BAT8001 injection for the treatment of HER2-positive advanced breast cancer - a multicenter, randomized, open-label, positive-controlled, superiority phase 3 clinical trial in china. | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04151329 | Clinical Status | Phase 1/2 | ||
| Clinical Description | Evaluation for the safety of BAT1306 and BAT8001 injection for the treatment of patients with HER2-positive advanced solid tumors phase 1/2a clinical trials of sexual, tolerability and pharmacokinetic characteristics. | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04189211 | Clinical Status | Phase 1 | ||
| Clinical Description | An open-label, dose escalation phase 1 clinical trial on safety, tolerability and pharmacokinetics of BAT8001 for injection in patients with HER2-positive solid tumors. | ||||
References
