Payload Information
General Information of This Payload
| Payload ID | PAY0ATCSD |
|||||
|---|---|---|---|---|---|---|
| Name | Maytansinoid derivative |
|||||
| Synonyms |
Maytansinoid derivative
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
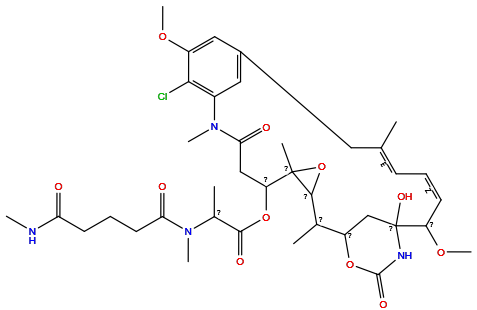
|
|||||
| Formula | C38H53ClN4O11 |
|||||
| Isosmiles | CNC(=O)CCCC(=O)N(C)C(C)C(=O)OC1CC(=O)N(C)c2cc(cc(OC)c2Cl)C/C(C)=C\C=C/C(OC)C2(O)CC(OC(=O)N2)C(C)C2OC12C |
|||||
| InChI |
InChI=1S/C38H53ClN4O11/c1-21-12-10-13-28(51-9)38(49)20-27(52-36(48)41-38)22(2)34-37(4,54-34)29(53-35(47)23(3)42(6)31(45)15-11-14-30(44)40-5)19-32(46)43(7)25-17-24(16-21)18-26(50-8)33(25)39/h10,12-13,17-18,22-23,27-29,34,49H,11,14-16,19-20H2,1-9H3,(H,40,44)(H,41,48)/b13-10-,21-12-
|
|||||
| InChIKey |
QTQHPYCMLRPHLI-USJWDNIUSA-N
|
|||||
| Pharmaceutical Properties | Molecule Weight |
777.312 |
Polar area |
185.57 |
||
Complexity |
776.3399362 |
xlogp Value |
3.4308 |
|||
Heavy Count |
54 |
Rot Bonds |
9 |
|||
Hbond acc |
11 |
Hbond Donor |
3 |
|||
Each Antibody-drug Conjugate Related to This Payload
