Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0ZOYQV
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Datopotamab deruxtecan
|
|||||
| Brand Name |
Datroway
|
|||||
| Synonyms |
DS 1062a; DS-1062; DS-1062, Dato-DXd; DS-1062a; Dato-DXd; Datopotamab deruxtecan
Click to Show/Hide
|
|||||
| Organization |
Daiichi Sankyo Co., Ltd.; AstraZeneca PLC; Baxter Oncology GmbH
|
|||||
| Drug Status |
Approved (FDA): Jan, 2025
|
|||||
| Indication |
In total 9 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
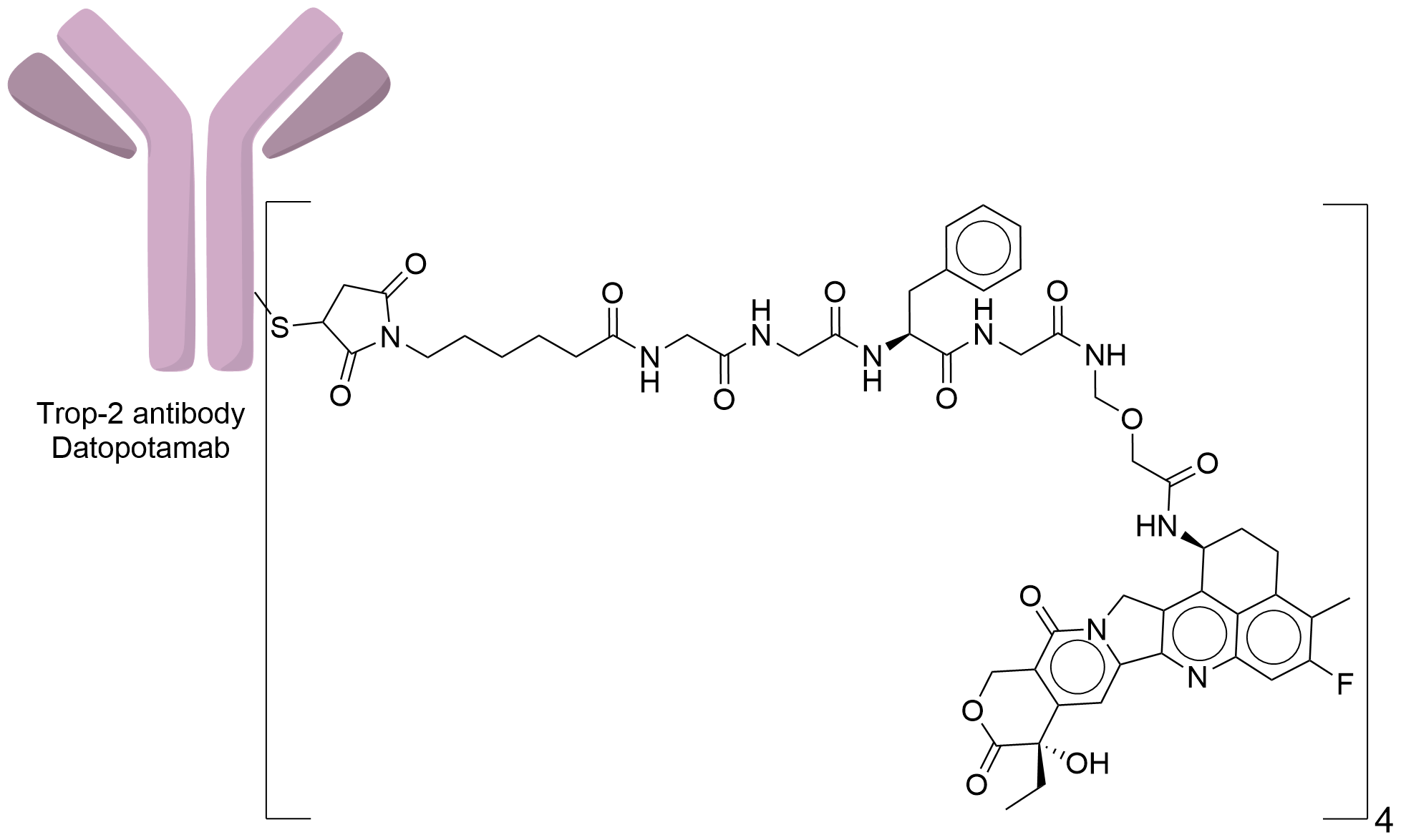
|
|||||
| Antibody Name |
Datopotamab
|
Antibody Info | ||||
| Antigen Name |
Tumor-associated calcium signal transducer 2 (TROP2)
|
Antigen Info | ||||
| Payload Name |
DX-8951 derivative (DXd)
|
Payload Info | ||||
| Therapeutic Target |
DNA topoisomerase 1 (TOP1)
|
Target Info | ||||
| Linker Name |
Mc-Gly-Gly-Phe-Gly
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
deruxtecan
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Cell Line-derived Xenograft Model
| Standard Type | Value | Units | Cell Line | Disease Model |
|---|---|---|---|---|
| Tumor Growth Inhibition value (TGI) |
≈ 96
|
%
|
NCI-N87 cells
|
Gastric tubular adenocarcinoma
|
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
24.00% (NSCLC, 4 mg/kg)
26.00% (NSCLC, 6 mg/kg) 24.00% (NSCLC, 8 mg/kg) 39.00% (TNBC, 6 mg/kg) |
|||
| Patients Enrolled |
Patients were unselected for TROP2 expression and had measurable disease per RECIST version 1.1; patients with stable/treated brain metastases were permitted.
|
||||
| Administration Dosage |
Dato-DXd 4 mg/kg (n=50), 6 mg/kg (n=50), or 8 mg/kg (n=80) intravenously every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03401385 | Clinical Status | Phase 1/2 | ||
| Clinical Description | Phase 1, two-part, multicenter, open-label, multiple dose, first-in-human study of DS-1062a in subjects with advanced solid tumors (TROPION-PanTumor01). | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
34.00
52.00% (in a subset nave to TOPO I-based ADCs) |
|||
| Patients Enrolled |
Unresectable a/mTNBC pts eligible for 1L treatment, regardless of PD-L1/TROP2 status.
|
||||
| Administration Dosage |
Intravenous Dato-DXd 6 mg/kg + durvalumab 1120 mg every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03742102 | Clinical Status | Phase 1/2 | ||
| Clinical Description | A phase 1b/2, 2-stage, open-label, multicenter study to determine the efficacy and safety of durvalumab (MEDI4736) + paclitaxel and durvalumab (MEDI4736) in combination with novel oncology therapies with or without paclitaxel for first-line metastatic triple negative breast cancer. | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 22.00% | High TROP2 expression (TROP2 +++) | ||
| Patients Enrolled |
Two hundred ten adults with locally advanced/metastatic non-small cell lung cancer (NSCLC).
|
||||
| Administration Dosage |
0.27-10 mg/kg Dato-DXd once every 3 weeks during escalation or 4 mg/kg Dato-DXd once every 3 weeks during expansion.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03401385 | Clinical Status | Phase 1 | ||
| Clinical Description | Phase 1, two-part, multicenter, open-label, multiple dose, first-in-human study of DS-1062a in subjects with advanced solid tumors (TROPION-PanTumor01). | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 23.80% | High TROP2 expression (TROP2 +++) | ||
| Patients Enrolled |
Two hundred ten adults with locally advanced/metastatic non-small cell lung cancer (NSCLC).
|
||||
| Administration Dosage |
0.27-10 mg/kg Dato-DXd once every 3 weeks during escalation or 8 mg/kg Dato-DXd once every 3 weeks during expansion.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03401385 | Clinical Status | Phase 1 | ||
| Clinical Description | Phase 1, two-part, multicenter, open-label, multiple dose, first-in-human study of DS-1062a in subjects with advanced solid tumors (TROPION-PanTumor01). | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 26.00% | High TROP2 expression (TROP2 +++) | ||
| Patients Enrolled |
Two hundred ten adults with locally advanced/metastatic non-small cell lung cancer (NSCLC).
|
||||
| Administration Dosage |
0.27-10 mg/kg Dato-DXd once every 3 weeks during escalation or 6 mg/kg Dato-DXd once every 3 weeks during expansion.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03401385 | Clinical Status | Phase 1 | ||
| Clinical Description | Phase 1, two-part, multicenter, open-label, multiple dose, first-in-human study of DS-1062a in subjects with advanced solid tumors (TROPION-PanTumor01). | ||||
| Primary Endpoint |
Patients receiving 6 mg/kg (n = 50), median duration on study, including follow-up, and median exposure were 13.30 and 3.50 months, respectively. The most frequent any-grade treatment-emergent adverse events (TEAEs) were nausea (64.00%), stomatitis (60.00%), and alopecia (42.00%).
|
||||
| Experiment 6 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
50.00% (doublet)
57.00% (triplet) |
|||
| Patients Enrolled |
Pts in escalation may have received 2 prior lines of therapy for a non-small cell lung cancer (NSCLC). Pts in expansion were primarily treatment (tx) naive (pts receiving Dato-DXd + pembro may have 1 prior Pt-based tx).
|
||||
| Administration Dosage |
Dato-DXd (4 or 6 mg/kg) + pembro 200 mg Pt-CT (cisplatin 75 mg/m2 or carboplatin AUC 5) every 21 days across 6 cohorts.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT04526691 | Clinical Status | Phase 1 | ||
| Clinical Description | Phase 1b, multicenter, open-label study of datopotamab deruxtecan (Dato-DXd) in combination with pembrolizumab with or without platinum chemotherapy in subjects with advanced or metastatic non-small cell lung cancer (TROPION-Lung02). | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [5] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05687266 | Clinical Status | Phase 3 | ||
| Clinical Description | A phase 3, randomised, open-label, multicentre, global study of datopotamab deruxtecan (Dato-DXd) in combination with durvalumab and carboplatin versus pembrolizumab in combination with platinum-based chemotherapy for the first-line treatment of patients with locally advanced or metastatic NSCLC without actionable genomic alterations (D926NC00001; AVANZAR). | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [6] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05629585 | Clinical Status | Phase 3 | ||
| Clinical Description | A phase 3 open-label, randomised study of datopotamab deruxtecan (DatoDXd) with or without durvalumab versus investigator's choice of therapy in patients with stage i-2i triple-negative breast cancer who have residual invasive disease in the breast and/or axillary lymph nodes at surgical resection following neoadjuvant systemic therapy (TROPION-Breast03). | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [7] | ||||
| Patients Enrolled |
Patients with previously untreated, advanced or metastatic non-squamous NSCLC with less than 50% programmed death-ligand (PD-L1) expression (tumor proportion score [TPS] < 50%) and without actionable genomic alterations.
|
||||
| Administration Dosage |
Arm A (datopotamab deruxtecan [6 mg/kg] plus pembrolizumab 200 mg IV plus platinum chemotherapy every three weeks), Arm B (datopotamab deruxtecan [6 mg/kg] plus pembrolizumab 200 mg IV every three weeks), and Arm C (pembrolizumab 200 mg IV plus pemetrexed [500 mg/m2] plus platinum chemotherapy every three weeks).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT05555732 | Clinical Status | Phase 3 | ||
| Clinical Description | A randomized phase 3 study of datopotamab deruxtecan (Dato-DXd) and pembrolizumab with or without platinum chemotherapy in subjects with no prior therapy for advanced or metastatic PD-L1 TPS <50% non-squamous non-small cell lung cancer without actionable genomic alterations (TROPION-Lung07). | ||||
| Experiment 10 Reporting the Activity Date of This ADC | [8] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05374512 | Clinical Status | Phase 3 | ||
| Clinical Description | A phase 3, open-label, randomised study of datopotamab deruxtecan (Dato-DXd) versus investigator's choice of chemotherapy in patients who are not candidates for PD-1/PD-L1 inhibitor therapy in first-line locally recurrent inoperable or metastatic triple-negative breast cancer (TROPION Breast02). | ||||
| Experiment 11 Reporting the Activity Date of This ADC | [9] | ||||
| Patients Enrolled |
Advanced non-small cell lung cancer (NSCLC).
|
||||
| Administration Dosage |
Dato-DXd 6 mg/kg plus pembrolizumab 200 mg every 3 weeks (arm 1) and pembrolizumab 200 mg every 3 weeks (arm 2).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT05215340 | Clinical Status | Phase 3 | ||
| Clinical Description | A randomized, open-label, phase 3 trial of Dato-DXd plus pembrolizumab vs pembrolizumab alone in treatment-nave subjects with advanced or metastatic PD-L1 high (TPS 50%) non-small cell lung cancer without actionable genomic alterations (TROPION-Lung08). | ||||
| Experiment 12 Reporting the Activity Date of This ADC | [10] | ||||
| Patients Enrolled |
Patients with inoperable or metastatic HR+/HER2 breast cancer.
|
||||
| Administration Dosage |
Dato-DXd 6 mg/kg IV Q3W or ICC (eribulin, capecitabine, vinorelbine, or gemcitabine).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT05104866 | Clinical Status | Phase 3 | ||
| Clinical Description | A phase-3, open-label, randomized study of Dato-DXd versus investigator's choice of chemotherapy (ICC) in participants with inoperable or metastatic HR-positive, HER2-negative breast cancer who have been treated with one or two prior lines of systemic chemotherapy (TROPION-Breast01). | ||||
| Experiment 13 Reporting the Activity Date of This ADC | [11] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04656652 | Clinical Status | Phase 3 | ||
| Clinical Description | Phase 3 randomized study of DS-1062a versus docetaxel in previously treated advanced or metastatic non-small cell lung cancer (TROPION-LUNG01). | ||||
| Experiment 14 Reporting the Activity Date of This ADC | [12] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05489211 | Clinical Status | Phase 2 | ||
| Clinical Description | A phase 2, multicentre, open-label, master protocol to evaluate the efficacy and safety of datopotamab deruxtecan (Dato-DXd) as monotherapy and in combination with anticancer agents in patients with advanced/metastatic solid tumours (TROPION-PanTumor03). | ||||
| Experiment 15 Reporting the Activity Date of This ADC | [13] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05061550 | Clinical Status | Phase 2 | ||
| Clinical Description | A phase 2, open-label, multicentre, randomised study of neoadjuvant and adjuvant treatment in patients with resectable, early-stage (2 to 2IB) non-small cell lung cancer (NeoCOAST-2). | ||||
| Experiment 16 Reporting the Activity Date of This ADC | [14] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04940325 | Clinical Status | Phase 2 | ||
| Clinical Description | Phase 2, open label study of DS-1062a, an anti-TROP-2-antibody-drug conjugate (ADC), in patients with advanced and/or unresectable non-small cell lung cancer (NSCLC), with biomarker analysis to characterize response to therapy. | ||||
| Experiment 17 Reporting the Activity Date of This ADC | [15] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04484142 | Clinical Status | Phase 2 | ||
| Clinical Description | Phase 2, single-arm, open-label study of DS-1062A in advanced or metastatic non-small cell lung cancer with actionable genomic alterations and progressed on or after applicable targeted therapy and platinum based chemotherapy (TROPION-Lung05). | ||||
| Experiment 18 Reporting the Activity Date of This ADC | [16] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03944772 | Clinical Status | Phase 2 | ||
| Clinical Description | A biomarker-directed phase 2 platform study in patients with advanced non-small lung cancer whose disease has progressed on first-line osimertinib therapy. | ||||
| Experiment 19 Reporting the Activity Date of This ADC | [17] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT01042379 | Clinical Status | Phase 2 | ||
| Clinical Description | I-SPY trial (investigation of serial studies to predict your therapeutic response with imaging and molecular analysis 2). | ||||
| Experiment 20 Reporting the Activity Date of This ADC | [18] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05460273 | Clinical Status | Phase 1/2 | ||
| Clinical Description | Phase 1/2, multicentre, open-label, multiple-cohort study of Dato-DXd in Chinese patients with advanced non-small-cell lung cancer, triple-negative breast cancer, gastric/gastroesophageal junction cancer, urothelial cancer, and other solid tumours (TROPION-PanTumor02). | ||||
| Experiment 21 Reporting the Activity Date of This ADC | [19] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04644068 | Clinical Status | Phase 1 | ||
| Clinical Description | A modular phase 1/2a, open-label, multicentre study to assess the safety, tolerability, pharmacokinetics, pharmacodynamics and preliminary efficacy of ascending doses of AZD5305 as monotherapy and in combination with anti-cancer agents in patients with advanced solid malignancies. | ||||
| Experiment 22 Reporting the Activity Date of This ADC | [20] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04612751 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1b, multicenter, 2-part, open-label study of datopotamab deruxtecan (Dato-DXd) in combination with immunotherapy with or without carboplatin in participants with advanced or metastatic non-small cell lung cancer (Tropion-Lung04). | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.00% (Day 21) | High TROP2 expression (TROP2+++) | ||
| Method Description |
Dato-DXd was intravenously administered at 10 mg/kg to NCI-N87 xenograft model mice. When the tumor volume reached approximately 150-300 mm3,the tumor-bearing mice were assigned to the vehicle control group,the treatment groups and the satellite sampling groups,and Dato-DXd or other test substances were administered intravenously once on day 0.
Click to Show/Hide
|
||||
| In Vivo Model | NCI-N87 cell line xenograft model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
References
