Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0YTRFZ
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Vandortuzumab vedotin
|
|||||
| Synonyms |
89Zr-DFO-MSTP2 109A (diagno stic); Anti-STEAP1-vc-MMAE; DSTP-3086S; DSTP3086S; RG-7450; RG7450, DSTP3 086S; Vandortuzumab vedotin
Click to Show/Hide
|
|||||
| Organization |
Genentech, Inc.; Roche Holding AG; Agensys, Inc.; Astellas Pharma, Inc.
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3 to 4
|
|||||
| Structure |
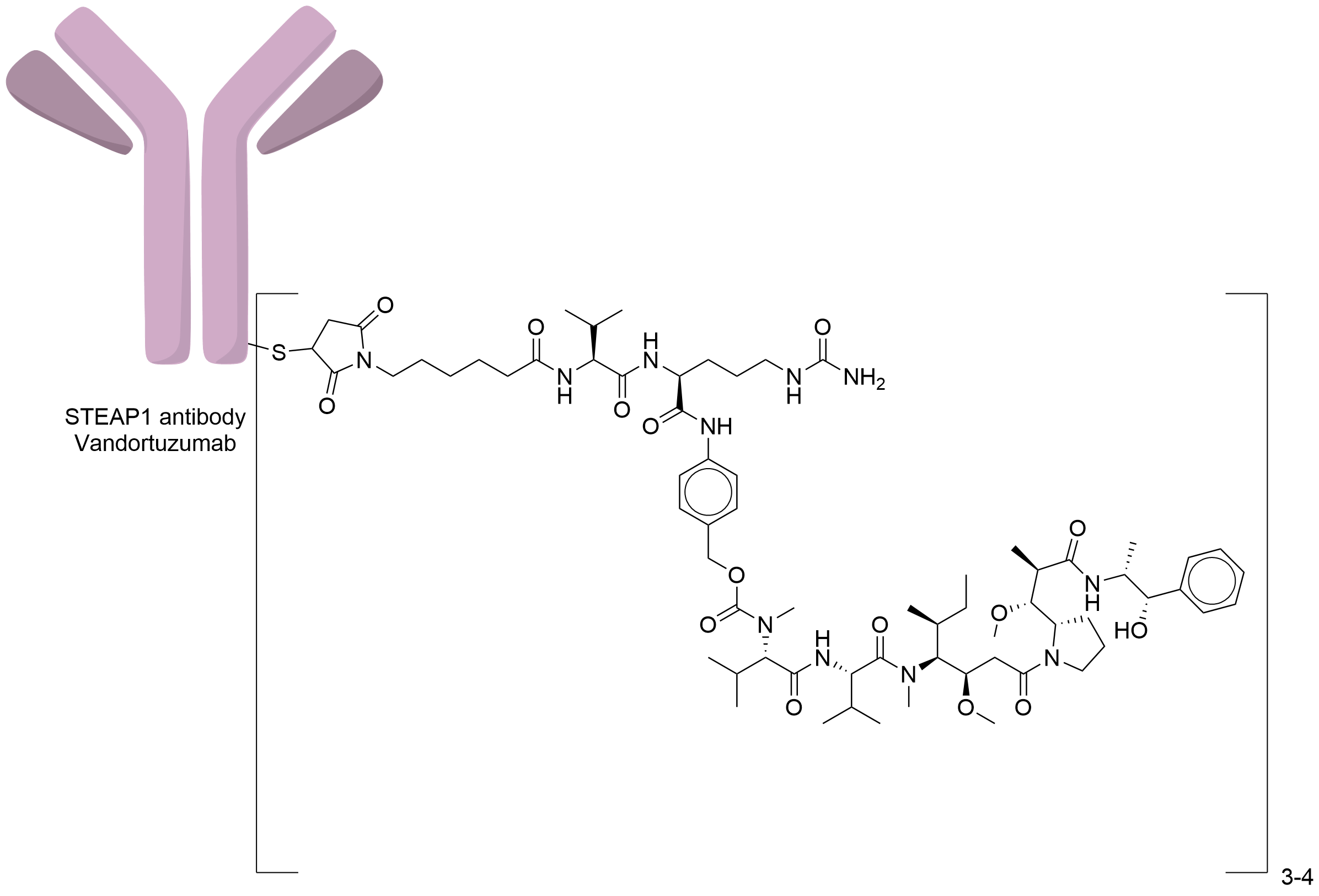
|
|||||
| Antibody Name |
Vandortuzumab
|
Antibody Info | ||||
| Antigen Name |
Metalloreductase STEAP1 (STEAP1)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
vedotin
|
|||||
| Puchem SID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Partial Response (PR) |
4.00%
|
|||
| Patients Enrolled |
Metastatic castration-resistant prostate cancer (CRPC).
|
||||
| Administration Dosage |
3 + 3 dose escalation study, 0.30 to 2.80 mg/kg intravenously given once every 3 weeks followed by cohort expansion at the recommended phase II dose or weekly (0.80 to 1.00 mg/kg).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01283373 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, open-label study of the safety and pharmacokinetics of escalating doses of DSTP3086S in patients with metastatic castration-resistant prostate cancer. | ||||
| Primary Endpoint |
DsTP3086S has acceptable safety at the recommended phase II dose level of 2.40 mg/kg once every 3 weeks.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT01283373 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, open-label study of the safety and pharmacokinetics of escalating doses of DSTP3086S in patients with metastatic castration-resistant prostate cancer. | ||||
References
