Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0VYGGK
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
BMS-986148
|
|||||
| Synonyms |
BMS 986148; BMS-986148; Mesothelin-ADC
Click to Show/Hide
|
|||||
| Organization |
Bristol Myers Squibb Co.
|
|||||
| Drug Status |
Phase 1/2 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3
|
|||||
| Structure |
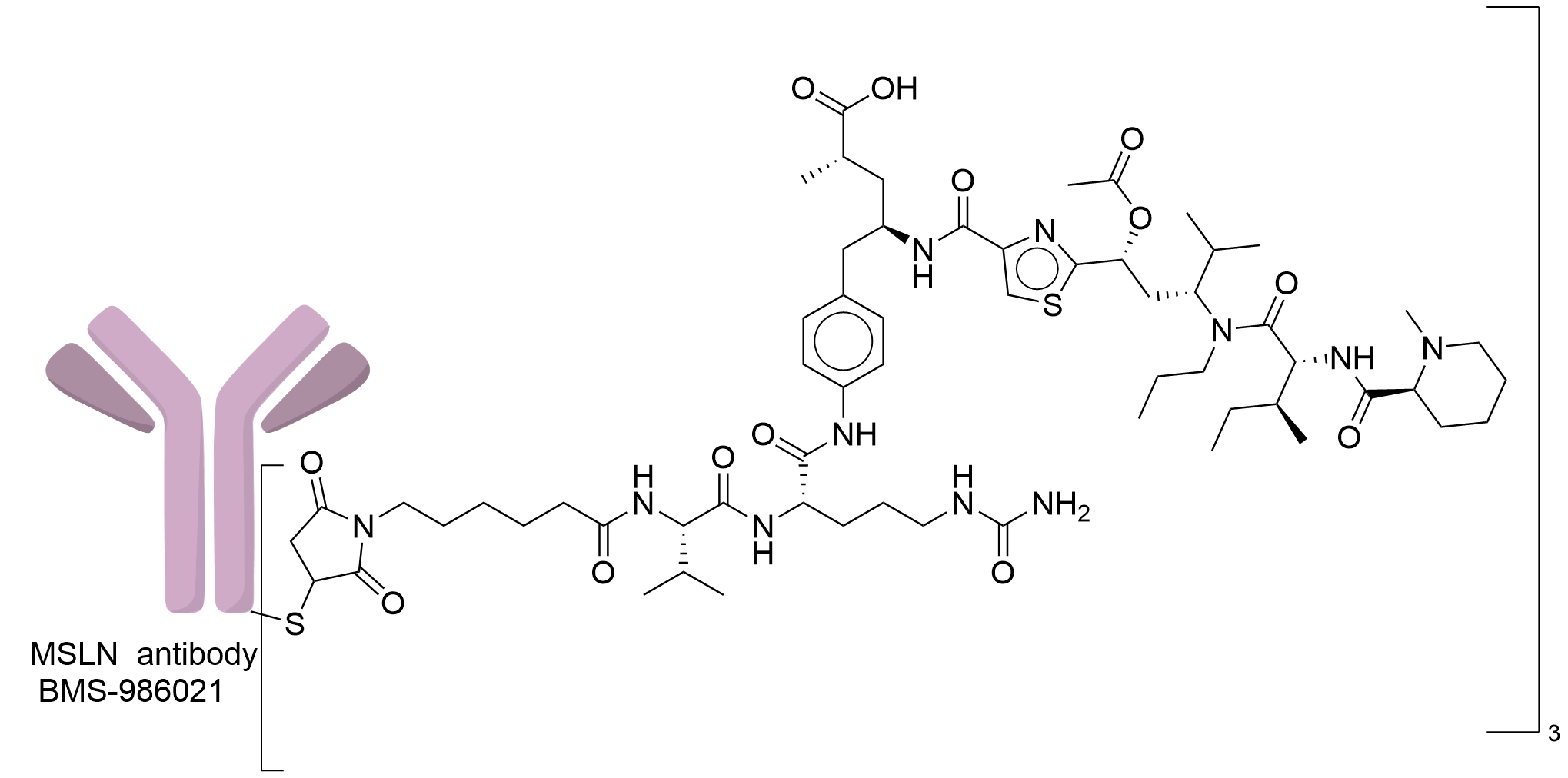
|
|||||
| Antibody Name |
BMS-986021
|
Antibody Info | ||||
| Antigen Name |
Mesothelin (MSLN)
|
Antigen Info | ||||
| Payload Name |
Tubulysin
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| TTD ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
2.00% (All escalation, BMS-986148 monotherapy)
6.00% (All expansion, BMS-986148 monotherapy) 4.00% (Mesothelioma expansion, BMS-986148 monotherapy) 9.00% (Ovarian expansion) 20.00% (All, BMS-986148 + nivolumab) 23.00% (Mesothelioma, BMS-986148 + nivolumab) |
|||
| Patients Enrolled |
Pleural or peritoneal mesothelioma (except sarcomatoid mesothelioma), ovarian cancer (except mucinous carcinoma), pancreatic cancer, gastric cancer, or non-small cell lung cancer (adenocarcinoma only).
|
||||
| Administration Dosage |
BMS-986148 monotherapy (0.1-1.6 mg/kg intravenously (i.v.) every 3 weeks or 0.4 or 0.6 mg/kg i.v. once weekly; n = 96) or BMS-986148 0.8 mg/kg + nivolumab 360 mg i.v. every 3 weeks (n = 30). The primary endpoint was safety and tolerability.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02341625 | Clinical Status | Phase 1/2a | ||
| Clinical Description | A phase 1/2a study of BMS-986148, a mesothelin directed antibody drug conjugate, in subjects with select advanced solid tumors. | ||||
| Primary Endpoint |
The MTD and the recommended dose for the part 2 monotherapy expansion is BMS-986148 1.20 mg/kg every 3 weeks dose level. The MTD in the combination expansion cohort is BMS-986148 0.80 mg/kg + nivolumab 360 mg every 3 weeks.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02341625 | Clinical Status | Phase 1/2 | ||
| Clinical Description | A phase 1/2a study of BMS-986148, a mesothelin directed antibody drug conjugate, in subjects with select advanced solid tumors. | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02884726 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 study of the safety and tolerability of BMS 986148 in subjects with advanced and/or metastatic solid tumors. | ||||
References
