Payload Information
General Information of This Payload
| Payload ID | PAY0PCTOE |
|||||
|---|---|---|---|---|---|---|
| Name | Tubulysin |
|||||
| Synonyms |
TUBULYSIN; 1943604-24-7; CHEMBL1288708; EX-A5466A; DLKUYSQUHXBYPB-NSSHGSRYSA-N; AKOS040740948; HY-128914; CS-0102166
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
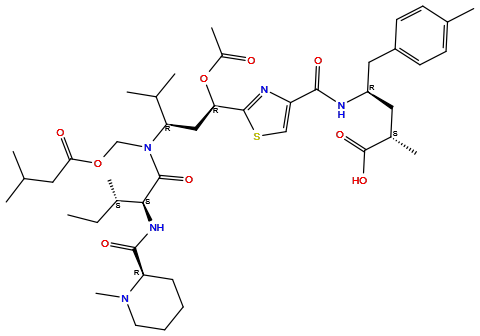
|
|||||
| Formula | C44H67N5O9S |
|||||
| Isosmiles | [H]OC(=O)[C@@]([H])(C([H])([H])[H])C([H])([H])[C@@]([H])(N([H])C(=O)c1nc([C@]([H])(OC(=O)C([H])([H])[H])C([H])([H])[C@@]([H])(N(C(=O)[C@@]([H])(N([H])C(=O)[C@]2([H])N(C([H])([H])[H])C([H])([H])C([H])([H])C([H])([H])C2([H])[H])[C@@]([H])(C([H])([H])[H])C([H])([H])C([H])([H])[H])C([H])([H])OC(=O)C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H])C([H])(C([H])([H])[H])C([H])([H])[H])sc1[H])C([H])([H])c1c([H])c([H])c(C([H])([H])[H])c([H])c1[H] |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C44H67N5O9S/c1-11-29(7)39(47-41(53)35-14-12-13-19-48(35)10)43(54)49(25-57-38(51)20-26(2)3)36(27(4)5)23-37(58-31(9)50)42-46-34(24-59-42)40(52)45-33(21-30(8)44(55)56)22-32-17-15-28(6)16-18-32/h15-18,24,26-27,29-30,33,35-37,39H,11-14,19-23,25H2,1-10H3,(H,45,52)(H,47,53)(H,55,56)/t29-,30-,33+,35+,36+,37+,39-/m0/s1
|
|||||
| InChIKey |
DLKUYSQUHXBYPB-NSSHGSRYSA-N
|
|||||
| IUPAC Name |
(2S,4R)-4-[[2-[(1R,3R)-1-acetyloxy-4-methyl-3-[3-methylbutanoyloxymethyl-[(2S,3S)-3-methyl-2-[[(2R)-1-methylpiperidine-2-carbonyl]amino]pentanoyl]amino]pentyl]-1,3-thiazole-4-carbonyl]amino]-2-methyl-5-(4-methylphenyl)pentanoic acid
|
|||||
| Pharmaceutical Properties | Molecule Weight |
842.113 |
Polar area |
184.54 |
||
Complexity |
841.4659497 |
xlogp Value |
6.31292 |
|||
Heavy Count |
59 |
Rot Bonds |
32 |
|||
Hbond acc |
12 |
Hbond Donor |
3 |
|||
The activity data of This Payload
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
BMS-986148 [Phase 1/2 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
2.00% (All escalation, BMS-986148 monotherapy)
6.00% (All expansion, BMS-986148 monotherapy) 4.00% (Mesothelioma expansion, BMS-986148 monotherapy) 9.00% (Ovarian expansion) 20.00% (All, BMS-986148 + nivolumab) 23.00% (Mesothelioma, BMS-986148 + nivolumab) |
|||
| Patients Enrolled |
Pleural or peritoneal mesothelioma (except sarcomatoid mesothelioma), ovarian cancer (except mucinous carcinoma), pancreatic cancer, gastric cancer, or non-small cell lung cancer (adenocarcinoma only).
|
||||
| Administration Dosage |
BMS-986148 monotherapy (0.1-1.6 mg/kg intravenously (i.v.) every 3 weeks or 0.4 or 0.6 mg/kg i.v. once weekly; n = 96) or BMS-986148 0.8 mg/kg + nivolumab 360 mg i.v. every 3 weeks (n = 30). The primary endpoint was safety and tolerability.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02341625 | Phase Status | Phase 1/2a | ||
| Clinical Description |
A phase 1/2a study of BMS-986148, a mesothelin directed antibody drug conjugate, in subjects with select advanced solid tumors.
|
||||
| Primary Endpoint |
The MTD and the recommended dose for the part 2 monotherapy expansion is BMS-986148 1.20 mg/kg every 3 weeks dose level. The MTD in the combination expansion cohort is BMS-986148 0.80 mg/kg + nivolumab 360 mg every 3 weeks.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02341625 | Phase Status | Phase 1/2 | ||
| Clinical Description |
A phase 1/2a study of BMS-986148, a mesothelin directed antibody drug conjugate, in subjects with select advanced solid tumors.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [5] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02884726 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 study of the safety and tolerability of BMS 986148 in subjects with advanced and/or metastatic solid tumors.
|
||||
References
